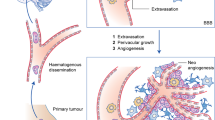
Opinion statement
Brain metastasis arising from breast cancer is associated with a poor prognosis. Various systemic chemotherapy and targeted therapies which are effective against breast cancer often fail to provide benefits against brain metastasis. This is mainly due to limited penetration of the therapies across the blood-brain barrier, and divergent evolution of brain metastasis compared to the primary tumor. Thus, brain metastasis is typically treated upfront with local therapies, such as surgery and radiation, followed by systemic therapies. Systemic therapies with CNS permeability are favored in patients with brain metastasis. This paper reviews various systemic therapy options for breast cancer brain metastasis.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Arvold ND, Oh KS, Niemierko A, Taghian AG, Lin NU, Abi-Raad RF, et al. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat. 2012;136(1):153–60. https://doi.org/10.1007/s10549-012-2243-x.
Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, Debled M, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991–1000. https://doi.org/10.1038/s41416-019-0619-y.
Kim YJ, Kim JS, Kim IA. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol. 2018;144(9):1803–16. https://doi.org/10.1007/s00432-018-2697-2.
Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(6):1663–74. https://doi.org/10.1158/1078-0432.CCR-06-2854.
Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–77. https://doi.org/10.1158/2159-8290.CD-15-0369.
Cosgrove N, Vareslija D, Keelan S, Elangovan A, Atkinson JM, Cocchiglia S, et al. Mapping molecular subtype specific alterations in breast cancer brain metastases identifies clinically relevant vulnerabilities. Nat Commun. 2022;13(1):514.
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40(5):492–516. https://doi.org/10.1200/JCO.21.02314.
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. The New England journal of medicine. 2006;355(26):2733–43. https://doi.org/10.1056/NEJMoa064320.
Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(4):1452–9. https://doi.org/10.1158/1078-0432.CCR-08-1080.
Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. The Lancet Oncology. 2013;14(1):64–71. This is a prospective trial showing the clinical benefit of lapatinib plus capecitabine in patients with HER2+ breast cancer with brain metastasis. https://doi.org/10.1016/S1470-2045(12)70432-1.
Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64(11):3958–65. https://doi.org/10.1158/0008-5472.CAN-03-2868.
Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, et al. TBCRC 022: a phase ii trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–9. This is the first prospective trial showing intracranial activity of neratinib plus capecitabine in patients with HER2+ breast cancer with brain metastasis. https://doi.org/10.1200/JCO.18.01511.
Saura C, Oliveira M, Feng YH, Dai MS, Chen SW, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with >/= 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–49. https://doi.org/10.1200/JCO.20.00147.
Hurvitz SA, Saura C, Oliveira M, Trudeau ME, Moy B, Delaloge S, et al. Efficacy of neratinib plus capecitabine in the subgroup of patients with central nervous system involvement from the NALA trial. Oncologist. 2021;26(8):e1327–e38. https://doi.org/10.1002/onco.13830.
Pheneger T, Bouhana K, Anderson D, Garrus J, Ahrendt K, Allen S, et al. Abstract #1795: in vitro and in vivo activity of ARRY-380: a potent, small molecule inhibitor of ErbB2. Cancer Research. 2009;69(9_Supplement):1795.
Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. The New England journal of medicine. 2020;382(7):597–609. https://doi.org/10.1056/NEJMoa1914609.
Curigliano G, Mueller V, Borges V, Hamilton E, Hurvitz S, Loi S, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Annals of oncology : official journal of the European Society for Medical Oncology. 2022;33(3):321–9. https://doi.org/10.1016/j.annonc.2021.12.005.
Lin NU, Murthy RK, Abramson V, Anders C, Bachelot T, Bedard P, et al. Abstract PD4-04: updated results of tucatinib vs placebo added to trastuzumab and capecitabine for patients with previously treated HER2-positive metastatic breast cancer with brain metastases (HER2CLIMB). Cancer Research. 2022;82(4_Supplement):PD4-04-PD4 This analysis from the landmark HER2CLIMB trial has established the clinical benefit and intracranial activity of a tucatinib-based regimen in not only stable/treated HER2+ breast cancer brain metastasis but also progressing brain metastasis.
Li X, Yang C, Wan H, Zhang G, Feng J, Zhang L, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51–61. https://doi.org/10.1016/j.ejps.2017.01.021.
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. The Lancet Oncology. 2021;22(3):351–60. https://doi.org/10.1016/S1470-2045(20)30702-6.
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. The Lancet Oncology. 2021;22(3):351–60. https://doi.org/10.1016/S1470-2045(20)30702-6.
Yan M, Ouyang Q, Sun T, Niu L, Yang J, Li L, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. The Lancet Oncology. 2022;23(3):353–61. https://doi.org/10.1016/S1470-2045(21)00716-6.
Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clinical Pharmacology & Therapeutics. 2010;87(5):586–92. https://doi.org/10.1038/clpt.2010.12.
Dijkers E, MNL-d H, Kosterink JG, Jager PL, Brouwers AH, Perk LR, et al. Characterization of 89Zr-trastuzumab for clinical HER2 immunoPET imaging. Journal of Clinical Oncology. 2007;25(18_suppl):3508. https://doi.org/10.1200/jco.2007.25.18_suppl.3508.
Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18(1):23–8. https://doi.org/10.1097/01.cad.0000236313.50833.ee.
Baculi RH, Suki S, Nisbett J, Leeds N, Groves M. Meningeal carcinomatosis from breast carcinoma responsive to trastuzumab. J Clin Oncol. 2001;19(13):3297–8. https://doi.org/10.1200/JCO.2001.19.13.3297.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. The New England journal of medicine. 2012;367(19):1783–91. https://doi.org/10.1056/NEJMoa1209124.
Montemurro F, Delaloge S, Barrios CH, Wuerstlein R, Anton A, Brain E, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial(). Annals of oncology : official journal of the European Society for Medical Oncology. 2020;31(10):1350–8. This analysis from the KAMILLA trial demonstrated intracranial activity of T-DM1 in HER2+ breast cancer brain metastasis. https://doi.org/10.1016/j.annonc.2020.06.020.
Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. The New England journal of medicine. 2022;386(12):1143–54. https://doi.org/10.1056/NEJMoa2115022.
Hurvitz S, Kim S-B, Chung W-P, Im S-A, Park YH, Hegg R, et al. Abstract GS3-01: trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Cancer Research. 2022;82(4_Supplement):GS3-01-GS3 This result from the DESTINY-Breast03 trial has shown remarkable intracranial activity of T-DXd in HER2+ breast cancer brain metastasis (stable/treated).
Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020:JCO2000775.
Kabraji S, Ni J, Sammons S, Van Swearingen AE, Wang Y, Pereslete AM, et al. Abstract PD4-05: preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases (BCBM). Cancer Research. 2022;82(4_Supplement):PD4-05-PD4.
Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, et al. 165MO trastuzumab-deruxtecan (T-DXd) in HER2-positive breast cancer patients (pts) with active brain metastases: primary outcome analysis from the TUXEDO-1 trial. Annals of Oncology. 2022;33:S198. https://doi.org/10.1016/j.annonc.2022.03.184.
Rosner D, Nemoto T, Lane WW. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986;58(4):832–9. https://doi.org/10.1002/1097-0142(19860815)58:4<832::AID-CNCR2820580404>3.0.CO;2-W.
Boogerd W, Dalesio O, Bais EM, Vandersande JJ. Response of brain metastases from breast-cancer to systemic chemotherapy. Cancer. 1992;69(4):972–80. https://doi.org/10.1002/1097-0142(19920215)69:4<972::AID-CNCR2820690423>3.0.CO;2-P.
Trudeau ME, Crump M, Charpentier D, Yelle L, Bordeleau L, Matthews S, et al. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada - Clinical Trials Group (NCIC-CTG). Annals of oncology : official journal of the European Society for Medical Oncology. 2006;17(6):952–6. https://doi.org/10.1093/annonc/mdl056.
Siena S, Crino L, Danova M, Del Prete S, Cascinu S, Salvagni S, et al. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Annals of oncology : official journal of the European Society for Medical Oncology. 2010;21(3):655–61. https://doi.org/10.1093/annonc/mdp343.
Trudeau ME, Crump M, Charpentier D, Yelle L, Bordeleau L, Matthews S, et al. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada - Clinical Trials Group (NCIC-CTG). Annals of Oncology. 2006;17(6):952–6. https://doi.org/10.1093/annonc/mdl056.
Melisko ME, Assefa M, Hwang J, DeLuca A, Park JW, Rugo HS. Phase II study of irinotecan and temozolomide in breast cancer patients with progressing central nervous system disease. Breast cancer research and treatment. 2019;177(2):401–8. https://doi.org/10.1007/s10549-019-05309-6.
Omuro AM, Raizer JJ, Demopoulos A, Malkin MG, Abrey LE. Vinorelbine combined with a protracted course of temozolomide for recurrent brain metastases: a phase I trial. J Neurooncol. 2006;78(3):277–80. https://doi.org/10.1007/s11060-005-9095-8.
Rivera E, Meyers C, Groves M, Valero V, Francis D, Arun B, et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 2006;107(6):1348–54. https://doi.org/10.1002/cncr.22127.
Cao KI, Lebas N, Gerber S, Levy C, Le Scodan R, Bourgier C, et al. Phase II randomized study of whole-brain radiation therapy with or without concurrent temozolomide for brain metastases from breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 2015;26(1):89–94. https://doi.org/10.1093/annonc/mdu488.
Cocconi G, Lottici R, Bisagni G, Bacchi M, Tonato M, Passalacqua R, et al. Combination therapy with platinum and etoposide of brain metastases from breast carcinoma. Cancer Invest. 1990;8(3-4):327–34.
Franciosi V, Cocconi G, Michiara M, Di Costanzo F, Fosser V, Tonato M, et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85(7):1599–605. https://doi.org/10.1002/(SICI)1097-0142(19990401)85:7<1599::AID-CNCR23>3.0.CO;2-#.
Lassman AB, Abrey LE, Shah GD, Panageas KS, Begemann M, Malkin MG, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78(3):255–60. https://doi.org/10.1007/s11060-005-9044-6.
Wang ML, Yung WK, Royce ME, Schomer DF, Theriault RL. Capecitabine for 5-fluorouracil-resistant brain metastases from breast cancer. American journal of clinical oncology. 2001;24(4):421–4. https://doi.org/10.1097/00000421-200108000-00026.
Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007;85(2):223–7. https://doi.org/10.1007/s11060-007-9409-0.
Adamo V, Ricciardi GRR, Giuffrida D, Scandurra G, Russo A, Blasi L, et al. Eribulin mesylate use as third-line therapy in patients with metastatic breast cancer (VESPRY): a prospective, multicentre, observational study. Ther Adv Med Oncol. 2019;11:1758835919895755. https://doi.org/10.1177/1758835919895755.
Goyal S, Puri T, Julka PK, Rath GK. Excellent response to letrozole in brain metastases from breast cancer. Acta Neurochir (Wien). 2008;150(6):613–4; discussion 4-5. https://doi.org/10.1007/s00701-008-1576-z.
Madhup R, Kirti S, Bhatt ML, Srivastava PK, Srivastava M, Kumar S. Letrozole for brain and scalp metastases from breast cancer—a case report. Breast. 2006;15(3):440–2. https://doi.org/10.1016/j.breast.2005.07.006.
Pors H, von Eyben FE, Sorensen OS, Larsen M. Longterm remission of multiple brain metastases with tamoxifen. J Neurooncol. 1991;10(2):173–7. https://doi.org/10.1007/BF00146879.
Rusz O, Koszo R, Dobi A, Csenki M, Valicsek E, Nikolenyi A, et al. Clinical benefit of fulvestrant monotherapy in the multimodal treatment of hormone receptor and HER2 positive advanced breast cancer: a case series. Onco Targets Ther. 2018;11:5459–63.
Tolaney SM, Sahebjam S, Le Rhun E, Bachelot T, Kabos P, Awada A, et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26(20):5310–9. https://doi.org/10.1158/1078-0432.CCR-20-1764.
Tolaney SM, Sahebjam S, Rhun EL, Bachelot T, Kabos P, Awada A, et al. Correction: a phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2021;27(5):1582. https://doi.org/10.1158/1078-0432.CCR-21-0193.
Brastianos PK, Kim AE, Wang N, Lee EQ, Ligibel J, Cohen JV, et al. Palbociclib demonstrates intracranial activity in progressive brain metastases harboring cyclin-dependent kinase pathway alterations. Nat Cancer. 2021;2(5):498–502. https://doi.org/10.1038/s43018-021-00198-5.
Batalini F, Moulder SL, Winer EP, Rugo HS, Lin NU, Wulf GM. Response of brain metastases from PIK3CA-mutant breast cancer to alpelisib. JCO Precis. Oncol. 2020;4. https://doi.org/10.1200/PO.19.00403.
Diossy M, Reiniger L, Sztupinszki Z, Krzystanek M, Timms KM, Neff C, et al. Breast cancer brain metastases show increased levels of genomic aberration-based homologous recombination deficiency scores relative to their corresponding primary tumors. Annals of oncology : official journal of the European Society for Medical Oncology. 2018;29(9):1948–54. https://doi.org/10.1093/annonc/mdy216.
Sambade MJ, Van Swearingen AED, McClure MB, Deal AM, Santos C, Sun K, et al. Efficacy and pharmacodynamics of niraparib in BRCA-mutant and wild-type intracranial triple-negative breast cancer murine models. Neurooncol Adv. 2019;1(1):vdz005. https://doi.org/10.1093/noajnl/vdz005.
Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. The New England journal of medicine. 2018;379(8):753–63. https://doi.org/10.1056/NEJMoa1802905.
Litton JK, Ettl J, Hurvitz SA, Martin M, Roche H, Lee K-H, et al. Clinical outcomes in patients (pts) with a history of central nervous system (CNS) metastases receiving talazoparib (TALA) or physician’s choice of chemotherapy (PCT) in the phase 3 EMBRACA trial. Journal of Clinical Oncology. 2021;39(15_suppl):1090. https://doi.org/10.1200/JCO.2021.39.15_suppl.1090.
Exman P, Mallery RM, Lin NU, Parsons HA. Response to olaparib in a patient with germline BRCA2 mutation and breast cancer leptomeningeal carcinomatosis. NPJ Breast Cancer. 2019;5:46.
Pascual T, Gonzalez-Farre B, Teixido C, Oleaga L, Oses G, Ganau S, et al. Significant clinical activity of olaparib in a somatic BRCA1-mutated triple-negative breast cancer with brain metastasis. Jco Precision. Oncology. 2019;3.
Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. The New England journal of medicine. 2021;384(16):1529–41. https://doi.org/10.1056/NEJMoa2028485.
Diéras V, Weaver R, Tolaney SM, Bardia A, Punie K, Brufsky A, et al. Abstract PD13-07: subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Research. 2021;81(4 Supplement):PD13-07-PD13-07.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–28. https://doi.org/10.1016/S0140-6736(20)32531-9.
Cortés J, Cescon DW, Rugo HS, Im SA, Md Yusof M, Gallardo C, et al. LBA16 KEYNOTE-355: final results from a randomized, double-blind phase III study of first-line pembrolizumab + chemotherapy vs placebo + chemotherapy for metastatic TNBC. Annals of Oncology. 2021;32:S1289–S90. https://doi.org/10.1016/j.annonc.2021.08.2089.
Sachdev JC, Munster P, Northfelt DW, Han HS, Ma C, Maxwell F, et al. Phase I study of liposomal irinotecan in patients with metastatic breast cancer: findings from the expansion phase. Breast cancer research and treatment. 2021;185(3):759–71. https://doi.org/10.1007/s10549-020-05995-7.
Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, et al. Survival of breast cancer patients with meningeal carcinomatosis. Annals of oncology : official journal of the European Society for Medical Oncology. 2010;21(11):2183–7. https://doi.org/10.1093/annonc/mdq232.
Rudnicka H, Niwinska A, Murawska M. Breast cancer leptomeningeal metastasis—the role of multimodality treatment. J Neurooncol. 2007;84(1):57–62. https://doi.org/10.1007/s11060-007-9340-4.
Heo MH, Cho YJ, Kim HK, Kim JY, Park YH. Isolated pachymeningeal metastasis from breast cancer: clinical features and prognostic factors. Breast. 2017;35:109–14. https://doi.org/10.1016/j.breast.2017.07.006.
Meattini I, Livi L, Saieva C, Franceschini D, Marrazzo L, Greto D, et al. Prognostic factors and clinical features in patients with leptominengeal metastases from breast cancer: a single center experience. J Chemother. 2012;24(5):279–84.
de Azevedo CR, Cruz MR, Chinen LT, Peres SV, Peterlevitz MA, de Azevedo Pereira AE, et al. Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. 2011;104(2):565–72. https://doi.org/10.1007/s11060-010-0524-y.
Jo JC, Kang MJ, Kim JE, Ahn JH, Jung KH, Gong G, et al. Clinical features and outcome of leptomeningeal metastasis in patients with breast cancer: a single center experience. Cancer Chemother Pharmacol. 2013;72(1):201–7. https://doi.org/10.1007/s00280-013-2185-y.
Niwinska A, Pogoda K, Michalski W, Kunkiel M, Jagiello-Gruszfeld A. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM). J Neurooncol. 2018;138(1):191–8. https://doi.org/10.1007/s11060-018-2790-z.
Morikawa A, Jordan L, Rozner R, Patil S, Boire A, Pentsova E, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clinical breast cancer. 2017;17(1):23–8. https://doi.org/10.1016/j.clbc.2016.07.002.
Chi Y, Shang M, Xu L, Gong H, Tao R, Song L, et al. Durable effect of pyrotinib and metronomic vinorelbine in HER2-positive breast cancer with leptomeningeal disease: a case report and literature review. Front Oncol. 2022;12:811919. https://doi.org/10.3389/fonc.2022.811919.
Yan F, Rinn KJ, Kullnat JA, Wu AY, Ennett MD, Scott EL, et al. Response of leptomeningeal metastasis of breast cancer with a HER2/neu activating variant to tucatinib: a case report. J Natl Compr Canc Netw. 2022:1–8.
Ricciardi GRR, Russo A, Franchina T, Schifano S, Mastroeni G, Santacaterina A, et al. Efficacy of T-DM1 for leptomeningeal and brain metastases in a HER2 positive metastatic breast cancer patient: new directions for systemic therapy - a case report and literature review. BMC Cancer. 2018;18(1):97.
Alder L, Trapani D, Van Swearingen A, Khasraw M, Anders C, Lin N, et al. Abstract 5257: durable clinical and radiographic responses in a series of patients with HER2+ breast cancer (BC) leptomeningeal disease (LMD) treated with trastuzumab deruxtecan (T-DXd). Cancer Research. 2022;82(12_Supplement):5257. https://doi.org/10.1158/1538-7445.AM2022-5257.
Stringer-Reasor EM, O'Brien BJ, Topletz-Erickson A, White JB, Lobbous M, Riley K, et al. Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast cancer. Journal of Clinical Oncology. 2021;39(15_suppl):1044.
Murthy RK, O'Brien B, Berry DA, Singareeka-Raghavendra A, Monroe MG, Johnson J, et al. Abstract PD4-02: safety and efficacy of a tucatinib-trastuzumab-capecitabine regimen for treatment of leptomeningeal metastasis (LM) in HER2-positive breast cancer: results from TBCRC049, a phase 2 non-randomized study. Cancer Research. 2022;82(4_Supplement):PD4-02-PD4 A novel prospective study demonstrating meaningful activity of tucatinib based regimen against HER2+ breast cancer leptomeningeal disease.
Kumthekar P, Tang SC, Brenner AJ, Kesari S, Piccioni DE, Anders C, et al. ANG1005, a brain-penetrating peptide-drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26(12):2789–99. https://doi.org/10.1158/1078-0432.CCR-19-3258.
Brastianos PK, Lee EQ, Cohen JV, Tolaney SM, Lin NU, Wang N, et al. Publisher correction: single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med. 2020;26(8):1309. https://doi.org/10.1038/s41591-020-0978-1.
Brastianos PK, Lee EQ, Cohen JV, Tolaney SM, Lin NU, Wang N, et al. Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med. 2020;26(8):1280–4. https://doi.org/10.1038/s41591-020-0918-0.
Tham YL, Hinckley L, Teh BS, Elledge R. Long-term clinical response in leptomeningeal metastases from breast cancer treated with capecitabine monotherapy: a case report. Clinical breast cancer. 2006;7(2):164–6. https://doi.org/10.3816/CBC.2006.n.028.
Shigekawa T, Takeuchi H, Misumi M, Matsuura K, Sano H, Fujiuchi N, et al. Successful treatment of leptomeningeal metastases from breast cancer using the combination of trastuzumab and capecitabine: a case report. Breast Cancer. 2009;16(1):88–92. https://doi.org/10.1007/s12282-008-0056-x.
Rogers LR, Remer SE, Tejwani S. Durable response of breast cancer leptomeningeal metastasis to capecitabine monotherapy. Neuro Oncol. 2004;6(1):63–4. https://doi.org/10.1215/S1152851703000334.
Giglio P, Tremont-Lukats IW, Groves MD. Response of neoplastic meningitis from solid tumors to oral capecitabine. J Neurooncol. 2003;65(2):167–72. https://doi.org/10.1023/B:NEON.0000003752.89814.ca.
Santa-Maria CA, Cimino-Mathews A, Moseley KF, Wolff AC, Blakeley JO, Connolly RM. Complete radiologic response and long-term survival with use of systemic high-dose methotrexate for breast cancer-associated leptomeningeal disease. Clinical breast cancer. 2012;12(6):445–9. https://doi.org/10.1016/j.clbc.2012.07.010.
Kapke JT, Schneidewend RJ, Jawa ZA, Huang CC, Connelly JM, Chitambar CR. High-dose intravenous methotrexate in the management of breast cancer with leptomeningeal disease: case series and review of the literature. Hematol Oncol Stem Cell Ther. 2019;12(4):189–93. https://doi.org/10.1016/j.hemonc.2019.08.008.
Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11(3):561–9. https://doi.org/10.1200/JCO.1993.11.3.561.
Byrnes DM, Vargas F, Dermarkarian C, Kahn R, Kwon D, Hurley J, et al. Complications of intrathecal chemotherapy in adults: single-institution experience in 109 consecutive patients. J Oncol. 2019;2019:4047617. https://doi.org/10.1155/2019/4047617.
Sinicrope KD, Barata P, Walker J, Tremont-Lukats IW, Groves M, Loghin M, et al. LPTO-09. Intrathecal topotecan for leptomeningeal metastasis in solid tumors: the MD Anderson experience. Neuro-oncology. Advances. 2019;1(Suppl 1):i8–i.
Kumthekar P, Lassman AB, Lin N, Grimm S, Gradishar W, Pentsova E, et al. LPTO-02. Intrathecal (IT) trastuzumab (T) for the treatment of leptomeningeal disease (LM) in patients (pts) with human epidermal receptor-2 positive (HER2+) cancer: a multicenter phase 1/2 study. Neurooncol Adv. 2019;1(Supplement_1):i6–i.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ajay Dhakal has received institutional funding from Celcuity Inc. and Puma biotechnology for research. Ajay Dhakal has received consulting fees from Gilead and MJH life sciences. Amanda E. D. Van Swearingen has nothing to disclose. Ruth O'Regan reports grants and personal fees from Novartis, personal fees from Pfizer, personal fees from Genomic Health, personal fees from Biotheranostics, personal fees from Lilly, personal fees from PUMA, personal fees from Genentech, personal fees from Immunomedics, and personal fees from Macrogenics, outside the submitted work. Carey K. Anders reports research funding from PUMA, Lilly, Merck, Seattle Genetics, Nektar, Tesaro, G1-Therapeutics, ZION, Novartis, and Pfizer. Carey K. Anders has received consulting fees from Genentech, Eisai, IPSEN, Seattle Genetics; Astra Zeneca, Novartis, Immunomedics, Elucida, and Athenex. Carey K. Anders has received royalties from UpToDate and Jones and Bartlett outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhakal, A., Van Swearingen, A.E.D., O’Regan, R. et al. Systemic Therapy Approaches for Breast Cancer Brain and Leptomeningeal Metastases. Curr. Treat. Options in Oncol. 23, 1457–1476 (2022). https://doi.org/10.1007/s11864-022-01011-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-022-01011-w