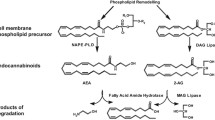
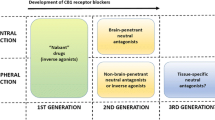
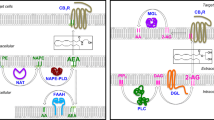
Abstract
Purpose of Review
The endocannabinoid (eCB) system, i.e. the receptors that respond to the psychoactive component of cannabis, their endogenous ligands and the ligand metabolic enzymes, is part of a larger family of lipid signals termed the endocannabinoidome (eCBome). We summarize recent discoveries of the roles that the eCBome plays within peripheral tissues in diabetes, and how it is being targeted, in an effort to develop novel therapeutics for the treatment of this increasingly prevalent disease.
Recent Findings
As with the eCB system, many eCBome members regulate several physiological processes, including energy intake and storage, glucose and lipid metabolism and pancreatic health, which contribute to the development of type 2 diabetes (T2D). Preclinical studies increasingly support the notion that targeting the eCBome may beneficially affect T2D.
Summary
The eCBome is implicated in T2D at several levels and in a variety of tissues, making this complex lipid signaling system a potential source of many potential therapeutics for the treatments for T2D.


Similar content being viewed by others
Abbreviations
- AcArGs:
-
1-acyl-sn-2-arachidonoyl-glycerols
- 2-AG:
-
2-arachidonoyl-glycerol
- 2-MAG:
-
2-mono-acyl-glycerol
- 2-OG:
-
2-oleoylglycerol
- 2-PG:
-
2-palmitoylglycerol
- abn-CBD:
-
abnormal cannabidiol
- AA:
-
arachidonic acid
- AEA:
-
arachidonoylethanolamide
- CB1/2:
-
cannabinoid receptor type-1/2
- THC:
-
D9-tetrahydrocannabinol
- THCV:
-
D9-Tetrahydrocannabivarin
- DAGL:
-
diacylglycerol lipase
- DHEA:
-
docohexanoylethanolamide
- eCB:
-
endocannabinoid
- eCBome:
-
endocannabinoidome
- FAAH:
-
fatty acid amide hydrolase
- GPR119:
-
G protein-coupled Receptor 119
- GPR55:
-
G protein-coupled Receptor 55
- GIP:
-
glucose-dependent insulinotropic polypeptide
- LEA:
-
linoleoylethanolamide
- MAGL:
-
monoacylglycerol lipase
- NAE:
-
N-acylethanolamine
- NAPE-PLD:
-
N-acyl-phosphatidylethanolamine-specific phospholipase D-like
- NArPEs:
-
N-arachidonoyl-phosphatidylethanolamines
- NAFLD:
-
nonalcoholic fatty liver disease
- OEA:
-
oleoylethanolamide
- PEA:
-
palmitoylethanolamide
- SEA:
-
stearoylethanolamide
- TRPV1:
-
transient receptor potential cation channel subfamily V member 1
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Matsuda LA, Young AC. Structure of a cannabinoid receptor and functional expression of the cloned eDNA. 1990;346:4.
Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5.
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9.
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90.
Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–24.
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7.
Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–8.
Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–305.
Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov. 2018;17:623–39.
Blüher M, Engeli S, Klöting N, Berndt J, Fasshauer M, Bátkai S, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–60.
Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–43.
van Eyk HJ, van Schinkel LD, Kantae V, Dronkers CEA, Westenberg JJM, de Roos A, et al. Caloric restriction lowers endocannabinoid tonus and improves cardiac function in type 2 diabetes. Nutr Diabetes [Internet]. 2018 [cited 2019 May 8];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5851430/
Fanelli F, Mezzullo M, Repaci A, Belluomo I, Ibarra Gasparini D, Di Dalmazi G, et al. Profiling plasma N-Acylethanolamine levels and their ratios as a biomarker of obesity and dysmetabolism. Mol Metab. 2018;14:82–94.
Fanelli F, Mezzullo M, Belluomo I, Di Lallo VD, Baccini M, Ibarra Gasparini D, et al. Plasma 2-arachidonoylglycerol is a biomarker of age and menopause related insulin resistance and dyslipidemia in lean but not in obese men and women. Mol Metab. 2017;6:406–15.
Matias I, Gonthier M-P, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and β-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–80.
Côté M, Matias I, Lemieux I, Petrosino S, Alméras N, Després J-P, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond). 2007;31:692–9.
de Martins CJM, Genelhu V, MMG P, BMJ C, Mangia RF, Aveta T, et al. Circulating endocannabinoids and the polymorphism 385C>A in fatty acid amide hydrolase (FAAH) gene May identify the obesity phenotype related to Cardiometabolic risk: A study conducted in a Brazilian population of complex interethnic admixture. PLoS One. 2015;10:e0142728.
Little TJ, Cvijanovic N, DiPatrizio NV, Argueta DA, Rayner CK, Feinle-Bisset C, et al. Plasma endocannabinoid levels in lean, overweight, and obese humans: relationships to intestinal permeability markers, inflammation, and incretin secretion. Am J Physiol Endocrinol Metab. 2018;315:E489–95.
Di Marzo V, Côté M, Matias I, Lemieux I, Arsenault BJ, Cartier A, et al. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2008;52:213.
Abdulnour J, Yasari S, Rabasa-Lhoret R, Faraj M, Petrosino S, Piscitelli F, et al. Circulating endocannabinoids in insulin sensitive vs. insulin resistant obese postmenopausal women. A MONET group study. Obesity. 2014;22:211–6.
Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect of high fat diets. Mol Cell Endocrinol. 2008;286:S66–78.
Grapov D, Adams SH, Pedersen TL, Garvey WT, Newman JW. Type 2 diabetes associated changes in the plasma non-esterified fatty acids, oxylipins and endocannabinoids. PLoS One. 2012;7:e48852.
Mallipedhi A, Prior SL, Dunseath G, Bracken RM, Barry J, Caplin S, et al. Changes in Plasma Levels of N-Arachidonoyl Ethanolamine and N-Palmitoylethanolamine following Bariatric Surgery in Morbidly Obese Females with Impaired Glucose Homeostasis. J Diabetes Res [Internet]. 2015 [cited 2019 May 8];2015. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4385619/
Gruden G, Barutta F, Kunos G, Pacher P. Role of the endocannabinoid system in diabetes and diabetic complications. Br J Pharmacol. 2016;173:1116–27.
Jourdan T, Djaouti L, Demizieux L, Gresti J, Vergès B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 2010;59:926–34.
Sidibeh CO, Pereira MJ, Lau Börjesson J, Kamble PG, Skrtic S, Katsogiannos P, et al. Role of cannabinoid receptor 1 in human adipose tissue for lipolysis regulation and insulin resistance. Endocrine. 2017;55:839–52.
Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100.
Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–40.
Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–67.
Silvestri C, Di Marzo V. The endocannabinoid system in energy Homeostasis and the Etiopathology of metabolic disorders. Cell Metab. 2013;17:475–90.
Ruiz de Azua I, Mancini G, Srivastava RK, Rey AA, Cardinal P, Tedesco L, et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J Clin Invest. 127:4148–62.
Moreno-Navarrete JM, Catalán V, Whyte L, Díaz-Arteaga A, Vázquez-Martínez R, Rotellar F, et al. The l-α-Lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes. 2012;61:281–91.
Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, et al. Activation of transient receptor potential Vanilloid Type-1 channel prevents Adipogenesis and obesity. Circ Res. 2007;100:1063–70.
Meadows A, Lee JH, Wu C-S, Wei Q, Pradhan G, Yafi M, et al. Deletion of G-protein-coupled receptor 55 promotes obesity by reducing physical activity. Int J Obes. 2016;40:417–24.
• Lipina C, Walsh SK, Mitchell SE, Speakman JR, Wainwright CL, Hundal HS. GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues. FASEB J. 2019;33:1299–312 Findings from this study demonstrate that GPR55, a widely expressed OEA and PEA receptor, is a positive regulator of adipogenesis and insulin action in skeletal muscle, adipose tissue and liver.
Alhouayek M, Masquelier J, Muccioli GG. Lysophosphatidylinositols, from cell membrane constituents to GPR55 ligands. Trends Pharmacol Sci. 2018;39:586–604.
Kang J-H, Tsuyoshi G, Han I-S, Kawada T, Kim YM, Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity. 2010;18:780–7.
Lee E, Jung DY, Kim JH, Patel PR, Hu X, Lee Y, et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015;29:3182–92.
Kuipers EN, Kantae V, Maarse BCE, van den Berg SM, van Eenige R, Nahon KJ, et al. High Fat Diet Increases Circulating Endocannabinoids Accompanied by Increased Synthesis Enzymes in Adipose Tissue. Front Physiol [Internet]. 2019 [cited 2019 May 15];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6335353/
Eckardt K, Sell H, Taube A, Koenen M, Platzbecker B, Cramer A, et al. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia. 2009;52:664–74.
Lindborg KA, Teachey MK, Jacob S, Henriksen EJ. Effects of in vitro antagonism of endocannabinoid-1 receptors on the glucose transport system in normal and insulin-resistant rat skeletal muscle. Diabetes Obes Metab. 2010;12:722–30.
•• Geurts L, Everard A, Van Hul M, Essaghir A, Duparc T, Matamoros S, et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nature Communications [Internet]. 2015 [cited 2018 Aug 3];6. Available from: http://www.nature.com/articles/ncomms7495. This study simultaneously demonstrates the complexity of NAPE-PLD enzyme activity in the regulation of eCBome mediator levels within adipocytes and its critical role in regulating adipose tissue physiology, obesity and whole-body glucose metabolism. Significantly, it also identifies the gut μB as a key factor in mediating the development of this phenotype, highlighting the importance of an adipose tissue eCBome - gut μB axis to metabolic health.
Kargl J, Balenga N, Parzmair GP, Brown AJ, Heinemann A, Waldhoer M. The cannabinoid receptor CB1 modulates the signaling properties of the Lysophosphatidylinositol receptor GPR55. J Biol Chem. 2012;287:44234–48.
Zelber-Sagi S, Azar S, Nemirovski A, Webb M, Halpern Z, Shibolet O, et al. Serum levels of endocannabinoids are independently associated with nonalcoholic fatty liver disease. Obesity. 2017;25:94–101.
Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–305.
Tedesco L, Valerio A, Dossena M, Cardile A, Ragni M, Pagano C, et al. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse White adipose tissue, muscle, and liver. Diabetes. 2010;59:2826–36.
Chanda D, Kim D-K, Li T, Kim Y-H, Koo S-H, Lee C-H, et al. Cannabinoid receptor type 1 (CB1R) signaling regulates hepatic gluconeogenesis via induction of endoplasmic reticulum-bound transcription factor cAMP-responsive element-binding protein H (CREBH) in primary hepatocytes. J Biol Chem. 2011;286:27971–9.
Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J, et al. Hepatic Cannabinoid Receptor-1 Mediates Diet-Induced Insulin Resistance via Inhibition of Insulin Signaling and Clearance in Mice. Gastroenterology. 2012;142:1218–1228.e1.
Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong W, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–9.
Jourdan T, Nicoloro SM, Zhou Z, Shen Y, Liu J, Coffey NJ, et al. Decreasing CB1 receptor signaling in Kupffer cells improves insulin sensitivity in obese mice. Molecular Metabolism. 2017;6:1517–28.
Li L, Li L, Chen L, Lin X, Xu Y, Ren J, et al. Effect of oleoylethanolamide on diet-induced nonalcoholic fatty liver in rats. J Pharmacol Sci. 2015;127:244–50.
Yang JW, Kim HS, Im JH, Kim JW, Jun DW, Lim SC, et al. GPR119: a promising target for nonalcoholic fatty liver disease. FASEB J. 2016;30:324–35.
Hiriart M, Velasco M, Larqué C, Diaz-Garcia CM. Metabolic syndrome and ionic channels in pancreatic beta cells. Vitam Horm. 2014;95:87–114.
González-Mariscal I, Egan JM. Endocannabinoids in the islets of Langerhans: the ugly, the bad, and the good facts. Am J Physiol Endocrinol Metab. 2018;315:E174–9.
Kim W, Doyle ME, Liu Z, Lao Q, Shin Y-K, Carlson OD, et al. Cannabinoids inhibit insulin receptor signaling in pancreatic β-cells. Diabetes. 2011;60:1198–209.
Li C, Vilches-Flores A, Zhao M, Amiel SA, Jones PM, Persaud SJ. Expression and function of monoacylglycerol lipase in mouse β-cells and human islets of Langerhans. Cell Physiol Biochem. 2012;30:347–58.
Malenczyk K, Keimpema E, Piscitelli F, Calvigioni D, Björklund P, Mackie K, et al. Fetal endocannabinoids orchestrate the organization of pancreatic islet microarchitecture. Proc Natl Acad Sci U S A. 2015;112:E6185–94.
Bermudez-Silva FJ, Sanchez-Vera I, Suárez J, Serrano A, Fuentes E, Juan-Pico P, et al. Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur J Pharmacol. 2007;565:207–11.
González-Mariscal I, Krzysik-Walker SM, Doyle ME, Liu Q-R, Cimbro R, Santa-Cruz Calvo S, et al. Human CB1 receptor isoforms, present in hepatocytes and β-cells, are involved in regulating metabolism. Sci Rep. 2016;6:33302.
Duvivier VF, Delafoy-Plasse L, Delion V, Lechevalier P, Le Bail J-C, Guillot E, et al. Beneficial effect of a chronic treatment with rimonabant on pancreatic function and beta-cell morphology in Zucker fatty rats. Eur J Pharmacol. 2009;616:314–20.
McKillop AM, Moran BM, Abdel-Wahab YHA, Gormley NM, Flatt PR. Metabolic effects of orally administered small-molecule agonists of GPR55 and GPR119 in multiple low-dose streptozotocin-induced diabetic and incretin-receptor-knockout mice. Diabetologia. 2016;59:2674–85.
Romero-Zerbo SY, Rafacho A, Díaz-Arteaga A, Suárez J, Quesada I, Imbernon M, et al. A role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. J Endocrinol. 2011;211:177–85.
Aichler M, Borgmann D, Krumsiek J, Buck A, MacDonald PE, Fox JEM, et al. N-acyl Taurines and Acylcarnitines Cause an Imbalance in Insulin Synthesis and Secretion Provoking β Cell Dysfunction in Type 2 Diabetes. Cell Metab. 2017;25:1334–1347.e4.
Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–75.
Di Marzo V, Capasso R, Matias I, Aviello G, Petrosino S, Borrelli F, et al. The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet. Br J Pharmacol. 2008;153:1272–80.
Capasso R, Orlando P, Pagano E, Aveta T, Buono L, Borrelli F, et al. Palmitoylethanolamide normalizes intestinal motility in a model of post-inflammatory accelerated transit: involvement of CB1 receptors and TRPV1 channels. Br J Pharmacol. 2014;171:4026–37.
Cluny NL, Keenan CM, Duncan M, Fox A, Lutz B, Sharkey KA. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone (SAB378), a peripherally restricted cannabinoid CB1/CB2 receptor agonist, inhibits gastrointestinal motility but has no effect on experimental colitis in mice. J Pharmacol Exp Ther. 2010;334:973–80.
Lin X-H, Yuece B, Li Y-Y, Feng Y-J, Feng J-Y, Yu L-Y, et al. A novel CB receptor GPR55 and its ligands are involved in regulation of gut movement in rodents. Neurogastroenterol Motil. 2011;23:862–e342.
Troy-Fioramonti S, Demizieux L, Gresti J, Muller T, Vergès B, Degrace P. Acute activation of cannabinoid receptors by anandamide reduces gastrointestinal motility and improves postprandial glycemia in mice. Diabetes. 2015;64:808–18.
Fu J, Kim J, Oveisi F, Astarita G, Piomelli D. Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R45–50.
Everard A, Plovier H, Rastelli M, Van Hul M, de Wouters d’Oplinter A, Geurts L, et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat Commun. 2019;10:457.
Stricker-Krongrad A, Beck B, Burlet C. Nitric oxide mediates hyperphagia of obese Zucker rats: relation to specific changes in the microstructure of feeding behavior. Life Sci. 1996;58:PL9–15.
Hankir MK, Seyfried F, Hintschich CA, Diep T-A, Kleberg K, Kranz M, et al. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017;25:335–44.
Overton HA, Babbs AJ, Doel SM, Fyfe MCT, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–75.
Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, et al. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–17.
Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058–66.
Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–8.
Igarashi M, DiPatrizio NV, Narayanaswami V, Piomelli D. Feeding-induced oleoylethanolamide mobilization is disrupted in the gut of diet-induced obese rodents. Biochim Biophys Acta. 1851;2015:1218–26.
Duszka K, Oresic M, Le May C, König J, Wahli W. PPARγ modulates long chain fatty acid processing in the intestinal epithelium. Int J Mol Sci. 2017;18.
Karwad MA, Couch DG, Theophilidou E, Sarmad S, Barrett DA, Larvin M, et al. The role of CB1 in intestinal permeability and inflammation. FASEB J. 2017;31:3267–77.
Alhamoruni A, Wright KL, Larvin M, O’Sullivan SE. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br J Pharmacol. 2012;165:2598–610.
. Karwad MA, Macpherson T, Wang B, Theophilidou E, Sarmad S, Barrett DA, et al. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARα. FASEB J. 2017;31:469–81 This in vitro study identifies a potential endogenous role for the eCBome mediators OEA and PEA in regulating intestinal permeability associated with inflammation. Both OEA, via TRPV1, and PEA, via PPARA, decrease cytokine-induced transepithelial permeability.
Acharya N, Penukonda S, Shcheglova T, Hagymasi AT, Basu S, Srivastava PK. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci U S A. 2017;114:5005–10.
Grunewald ZI, Lee S, Kirkland R, Ross M, de La Serre CB. Cannabinoid receptor type-1 partially mediates metabolic endotoxemia-induced inflammation and insulin resistance. Physiol Behav. 2019;199:282–91.
Couch DG, Tasker C, Theophilidou E, Lund JN, O’Sullivan SE. Cannabidiol and palmitoylethanolamide are anti-inflammatory in the acutely inflamed human colon. Clin Sci. 2017;131:2611–26.
Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, et al. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2014;63:1300–12.
Després J-P, Golay A, Sjöström L. Effects of Rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;14.
Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S, RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97.
Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–75.
Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF, RIO-Diabetes Study Group. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–72.
Sam AH, Salem V, Ghatei MA. Rimonabant: From RIO to Ban. J Obes [Internet]. 2011 [cited 2019 May 11];2011. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3136184/
Silvestri C, Marzo VD. Second generation CB1 receptor blockers and other inhibitors of peripheral endocannabinoid overactivity and the rationale of their use against metabolic disorders. Expert Opin Investig Drugs. 2012;21:1309–22.
Tam J, Hinden L, Drori A, Udi S, Azar S, Baraghithy S. The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. Eur J Intern Med. 2018;49:23–9.
Cinar R, Godlewski G, Liu J, Tam J, Jourdan T, Mukhopadhyay B, et al. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology. 2014;59:143–53.
Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16:167–79.
Romero-Zerbo SY, Ruz-Maldonado I, Espinosa-Jiménez V, Rafacho A, Gómez-Conde AI, Sánchez-Salido L, et al. The cannabinoid ligand LH-21 reduces anxiety and improves glucose handling in diet-induced obese pre-diabetic mice. Sci Rep. 2017;7:3946.
Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–66.
Ma H, Zhang G, Mou C, Fu X, Chen Y. Peripheral CB1 Receptor Neutral Antagonist, AM6545, Ameliorates Hypometabolic Obesity and Improves Adipokine Secretion in Monosodium Glutamate Induced Obese Mice. Front Pharmacol [Internet]. 2018 [cited 2019 May 12];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5869198/
Cluny NL, Chambers AP, Vemuri VK, Wood JT, Eller LK, Freni C, et al. The neutral cannabinoid CB1 receptor antagonist A M4113 regulates body weight through changes in energy intake in the rat. Pharmacol Biochem Behav. 2011;97:537–43.
Agudo J, Martin M, Roca C, Molas M, Bura AS, Zimmer A, et al. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia. 2010;53:2629–40.
Deveaux V, Cadoudal T, Ichigotani Y, Teixeira-Clerc F, Louvet A, Manin S, et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, Insulin Resistance and Hepatic Steatosis. PLOS ONE. 2009;4:e5844.
Zhang X, Gao S, Niu J, Li P, Deng J, Xu S, et al. Cannabinoid 2 receptor agonist improves systemic sensitivity to insulin in high-fat diet/Streptozotocin-induced diabetic mice. CPB. 2016;40:1175–85.
Imtiaz S, Rehm J. The relationship between cannabis use and diabetes: results from the National Epidemiologic Survey on alcohol and related conditions III. Drug Alcohol Rev. 2018;37:897–902.
Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med. 2013;126:583–9.
Clark T, Jessica Jones, Hall A, Tabner S, Kmiec R. Theoretical Explanation for Reduced Body Mass Index and Obesity Rates in Cannabis Users. 2018 [cited 2019 Jan 8]; Available from: http://www.preprints.org/manuscript/201807.0197/v1
Roger Pertwee, Maria Grazia Cascio. Chapter 6: Known Pharmacological Actions of Delta-9-Tetrahydrocannabinol and of Four Other Chemical Constituents of Cannabis that Activate Cannabinoid Receptors. In: Roger Pertwee, editor. Handbook of Cannabis [Internet]. First Edition. Oxford University Press; 2014. p. 115–136. Available from: https://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780199662685.001.0001/acprof-9780199662685-chapter-6
Wargent ET, Zaibi MS, Silvestri C, Hislop DC, Stocker CJ, Stott CG, et al. The cannabinoid Δ9-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutr Diabetes. 2013;3:e68.
Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, et al. Efficacy and safety of Cannabidiol and Tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, Parallel Group Pilot Study. Diabetes Care. 2016;39:1777–86.
Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13:36–49.
Moriconi A, Cerbara I, Maccarrone M, Topai A. GPR55: current knowledge and future perspectives of a purported “Type-3” cannabinoid receptor. Curr Med Chem. 2010;17:1411–29.
McKillop AM, Moran BM, Abdel-Wahab YHA, Flatt PR. Evaluation of the insulin releasing and antihyperglycaemic activities of GPR55 lipid agonists using clonal beta-cells, isolated pancreatic islets and mice. Br J Pharmacol. 2013;170:978–90.
Yang JW, Kim HS, Choi Y-W, Kim Y-M, Kang KW. Therapeutic application of GPR119 ligands in metabolic disorders. Diabetes Obes Metab. 2018;20:257–69.
Ritter K, Buning C, Halland N, Pöverlein C, Schwink L. G protein-coupled receptor 119 (GPR119) agonists for the treatment of diabetes: recent Progress and prevailing challenges. J Med Chem. 2016;59:3579–92.
Ohishi T, Yoshida S. The therapeutic potential of GPR119 agonists for type 2 diabetes. Expert Opin Investig Drugs. 2012;21:321–8.
Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, et al. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–17.
Mandøe MJ, Hansen KB, Windeløv JA, Knop FK, Rehfeld JF, Rosenkilde MM, et al. Comparing olive oil and C4-dietary oil, a prodrug for the GPR119 agonist, 2-oleoyl glycerol, less energy intake of the latter is needed to stimulate incretin hormone secretion in overweight subjects with type 2 diabetes. Nutr Diabetes. 2018;8:2.
Effect of Dietary Oils as G-protein-coupled Receptor Agonists on Glucose Tolerance - Full Text View - ClinicalTrials.gov [Internet]. [cited 2019 May 13]. Available from: https://clinicaltrials.gov/ct2/show/NCT03774095
Song J-X, Ren H, Gao Y-F, Lee C-Y, Li S-F, Zhang F, et al. Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice. Frontiers in Physiology [Internet]. 2017 [cited 2018 Jul 25];8. Available from: http://journal.frontiersin.org/article/10.3389/fphys.2017.00602/full
Kang J-H, Tsuyoshi G, Le Ngoc H, Kim H-M, Tu TH, Noh H-J, et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J Med Food. 2011;14:310–5.
Kang C, Wang B, Kaliannan K, Wang X, Lang H, Hui S, et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. mBio. 2017;8:e00470–17.
Wang P, Yan Z, Zhong J, Chen J, Ni Y, Li L, et al. Transient receptor potential Vanilloid 1 activation enhances gut glucagon-like Peptide-1 secretion and improves glucose Homeostasis. Diabetes. 2012;61:2155–65.
Kroff J, Hume DJ, Pienaar P, Tucker R, Lambert EV, Rae DE. The metabolic effects of a commercially available chicken peri-peri (African bird’s eye chilli) meal in overweight individuals. Br J Nutr. 2017;117:635–44.
Urbina SL, Roberts MD, Kephart WC, Villa KB, Santos EN, Olivencia AM, et al. Effects of twelve weeks of capsaicinoid supplementation on body composition, appetite and self-reported caloric intake in overweight individuals. Appetite. 2017;113:264–73.
Touska F, Marsakova L, Teisinger J, Vlachova V. A “cute” desensitization of TRPV1. Curr Pharm Biotechnol. 2011;12:122–9.
Gram DX, Hansen AJ. Inhibition of the activity of the capsaicin receptor in the treatment of obesity or obesity-related diseases and disorders [Internet]. 2011 [cited 2019 May 14]. Available from: https://patents.google.com/patent/US7879866B2/en
Clinical Trials Register [Internet]. [cited 2019 May 14]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-003843-12/DK#E
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103.
Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev. 2018;31:35–51.
Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, et al. The endocannabinoid system links gut microbiota to adipogenesis. Molecular Systems Biology [Internet]. 2010 [cited 2018 Aug 3];6. Available from: http://msb.embopress.org/cgi/doi/10.1038/msb.2010.46
Geurts L, Lazarevic V, Derrien M, Everard A, Van Roye M, Knauf C, et al. Altered Gut Microbiota and Endocannabinoid System Tone in Obese and Diabetic Leptin-Resistant Mice: Impact on Apelin Regulation in Adipose Tissue. Front Microbiol [Internet]. 2011 [cited 2018 Aug 3];2. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2011.00149/full
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. PNAS. 2013;110:9066–71.
Evaluation of the Effects Associated With the Administration of Akkermansia Muciniphila on Parameters of Metabolic Syndrome - Full Text View - ClinicalTrials.gov [Internet]. [cited 2019 May 14]. Available from: https://clinicaltrials.gov/ct2/show/NCT02637115
Brodie JS, Di Marzo V, Guy GW. Polypharmacology shakes hands with complex Aetiopathology. Trends Pharmacol Sci. 2015;36:802–21.
Piscitelli F, Carta G, Bisogno T, Murru E, Cordeddu L, Berge K, et al. Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice. Nutr Metab (Lond). 2011;8:51.
Demizieux L, Piscitelli F, Troy-Fioramonti S, Iannotti FA, Borrino S, Gresti J, et al. Early low-fat diet enriched with linolenic acid reduces liver endocannabinoid tone and improves late glycemic control after a high-fat diet challenge in mice. Diabetes. 2016;65:1824–37.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alain Veilleux declares no conflict of interest.
Vincenzo Di Marzo reports grants from GW Pharmaceuticals.
Cristoforo Silvestri reports he was a previous employee of GW Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Obesity
Rights and permissions
About this article
Cite this article
Veilleux, A., Di Marzo, V. & Silvestri, C. The Expanded Endocannabinoid System/Endocannabinoidome as a Potential Target for Treating Diabetes Mellitus. Curr Diab Rep 19, 117 (2019). https://doi.org/10.1007/s11892-019-1248-9
Published:
DOI: https://doi.org/10.1007/s11892-019-1248-9