Abstract
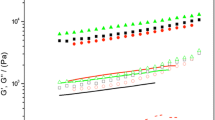
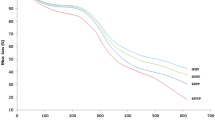
High pressure (HP, 200, 400, and 600 MPa)- and heat (60, 80, and 100 °C)-induced gelation, aggregation, and structural conformations of rapeseed protein isolate (RPI) were characterized using gel permeation–size-exclusion chromatography, differential scanning calorimetry, and circular dichroism (CD) techniques. HP treatments significantly (p < 0.05) increased the content of soluble protein aggregates and surface hydrophobicity of RPI. In contrast, heat treatments at 80 and 100 °C led to significant (p < 0.05) decreases in the amount of soluble protein aggregates. At pressure treatment of 200 MPa, there was a significant (p < 0.05) increase in free sulfhydryl group content of RPI, whereas 400- and 600-MPa treatments as well as temperature treatments (60–100 °C) caused significant decreases. Protein denaturation temperature was increased by about 6 °C by HP and heat treatments. The far-UV CD spectra revealed increases in α-helix content of RPI after HP treatments with 400 MPa producing the most increase. Near-UV data showed that HP and heat treatments of RPI led to increasing interactions among the aromatic amino acids (evidence of protein aggregation), and between aromatic amino acids and the hydrophilic environment, which indicates protein unfolding. Least gelation concentration of RPI was significantly (p < 0.05) reduced by HP and heat treatments, but HP-treated RPI produced gels with better textural properties (hardness increased from ~7.7 to 81.1 N, while springiness increased from ~0.37 to 0.99). Overall, pressure treatments (200–600 MPa) were better than heat treatments (60–100 °C) to modify the structure and improve gelation properties of RPI.





Similar content being viewed by others
References
Ahmed, J., Ayad, A., Ramaswamy, H. S., Am, I., & Shao, Y. (2007). Dynamic viscoelastic behavior of high pressure treated soybean protein isolate dispersions. International Journal of Food Properties, 10, 397–411.
Ahmed, J., Ramaswamy, H. S., Kasapis, S., & Boye, J. I. (2010). Novel food processing: effects on rheological and functional properties (pp. 226–229). Boca Raton: CRC.
Aluko, R. E., Mofolasayo, O. A., & Watts, B. A. (2009). Emulsifying and foaming properties of commercial yellow pea (Pisum sativum L.) seed flours. Journal of Agricultural and Food Chemistry, 57, 9793–9800.
AOAC. (1990). Official methods of analysis (15th ed., Vol. I and II). Washington, DC: Association of Official Analytical Chemists, Inc.
Balny, C. (2004). Pressure effects on weak interactions in biological systems. Journal of Physics: Condensed Matter, 16, 1245–1253.
Barba, F. J., Esteve, M. J., & Frigola, A. (2012). High pressure treatment effect on physicochemical and nutritional properties of fluid foods during storage: a review. Comprehensive Reviews in Food Science and Food Safety, 11, 307–322.
Barbin, D. F., Natsch, A., & Muller, K. (2011). Improvement of functional properties of rapeseed protein concentrates produced via alcoholic processes by thermal and mechanical treatments. Journal of Food Processing and Preservation, 35, 369–375.
Bérot, S., Compoint, J. P., Larré, C., Malabat, C., & Guéguen, J. (2005). Large scale purification of rapeseed proteins (Brassica napus L.). Journal of Chromatography B, 818, 35–42.
Beveridge, T., Toma, S. J., & Nakai, S. (1974). Determination of SH-groups and SS-groups in some food proteins using ellmans reagent. Journal of Food Science, 39, 49–51.
Boonyaratanakornkit, B. B., Park, C. B., & Clark, D. S. (2002). Pressure effects on intra- and intermolecular interactions within proteins. Biochimica et Biophysica Acta-Protein Structure and Molecular Enzymology, 1595, 235–249.
Chabanon, G., Chevalot, I., Framboisier, X., Chenu, S., & Marc, I. (2007). Hydrolysis of rapeseed protein isolates: kinetics, characterization and functional properties of hydrolysates. Process Biochemistry, 42, 1419–1428.
Condes, M. C., Speroni, F., Mauri, A., & Anon, M. C. (2012). Physicochemical and structural properties of amaranth protein isolates treated with high pressure. Innovative Food Science & Emerging Technologies, 14, 11–17.
Devi, A. F., Buckow, R., Hemar, Y., & Kasapis, S. (2013). Structuring dairy systems through high pressure processing. Journal of Food Engineering, 114, 106–122.
Dumoulin, M., Ozawa, S., & Hayashi, R. (1998). High pressure, a unique tool for food texturization. Food Science and Technology International (Tokyo), 4, 99–113.
Fahey, J. W., Zalcmann, A. T., & Talalay, P. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry, 56, 5–51.
Fleddermann, M., Fechner, A., Rößler, A., Bähr, M., Pastor, A., Liebert, F., et al. (2012). Nutritional evaluation of rapeseed protein compared to soy protein for quality, plasma amino acids, and nitrogen balance—a randomized cross-over intervention study in humans. Clinical Nutrition. doi:10.1016/j.clnu.2012.11.005.
Hayakawa, I., Link, Y.-Y., & Linko, P. (1996). Mechanism of high pressure denaturation of proteins. LWT- Food Science and Technology, 29, 756–762.
Jang, S. A., Shin, Y. J., & Song, K. B. (2011a). Effect of rapeseed protein–gelatin film containing grapefruit seed extract on ‘Maehyang’ strawberry quality. International Journal of Food Science and Technology, 46, 620–625.
Jang, S. A., Lim, G. O., & Bin Song, K. (2011b). Preparation and mechanical properties of edible rapeseed protein films. Journal of Food Science, 76, C218–C223.
Keim, S., & Hinrichs, J. (2004). Influence of stabilizing bonds on the texture properties of high-pressure-induced whey protein gels. International Dairy Journal, 14, 355–363.
Kelly, S. M., Jess, T. J., & Price, N. C. (2005). How to study proteins by circular dichroism. Biochimica et Biophysica Acta−Proteins and Proteomics, 1751, 119–139.
Krause, J. P., & Schwenke, K. D. (2001). Behaviour of a protein isolate from rapeseed (Brassica napus) and its main protein components—globulin and albumin—at air/solution and solid interfaces, and in emulsions. Colloids and Surfaces. B, Biointerfaces, 21, 29–36.
Li, H., Zhu, K., Zhou, H., & Peng, W. (2011). Effects of high hydrostatic pressure on some functional and nutritional properties of soy protein isolate for infant formula. Journal of Agricultural and Food Chemistry, 59, 12028–12036.
Li, H., Zhu, K., Zhou, H., & Peng, W. (2012). Effects of high hydrostatic pressure treatment on allergenicity and structural properties of soybean protein isolate for infant formula. Food Chemistry, 132, 808–814.
Lobley, A., Whitmore, L., & Wallace, B. A. (2002). DICHROWEB: an interactive website for the analysis of protein secondary structure from circular dichroism spectra. Bioinformatics, 18, 211–212.
Markwell, M. A. K., Haas, S. M., Bieber, L. L., & Tolbert, N. E. (1978). Modification of lowry procedure to simplify protein determination in membrane and lipoprotein samples. Analytical Biochemistry, 87, 206–210.
Molina, E., & Ledward, D. A. (2003). Effects of combined high-pressure and heat treatment on the textural properties of soya gels. Food Chemistry, 80, 367–370.
O’Kane, F. E., Vereijken, J. M., Gruppen, H., & Van Boekel, M. A. J. S. (2005). Gelation behavior of protein isolates extracted from 5 cultivars of Pisum sativum L. Journal of Food Science, 70, C132–C137.
Omoni, A. O., & Aluko, R. E. (2006). Effect of cationic flaxseed protein hydrolysate fractions on the in vitro structure and activity of calmodulin-dependent endothelial nitric oxide synthase. Molecular Nutrition & Food Research, 50, 958–966.
Pinterits, A., & Arntfield, S. D. (2008). Improvement of canola protein gelation properties through enzymatic modification with transglutaminase. LWT- Food Science and Technology, 41, 128–138.
Qin, Z., Guo, X., Lin, Y., Chen, J., Liao, X., Hu, X., et al. (2012). Effects of high hydrostatic pressure on physicochemical and functional properties of walnut (Juglans regia L.) protein isolate. Journal of the Science of Food and Agriculture. doi:10.1002/jsfa.5857.
Raikos, V. (2010). Effect of heat treatment on milk protein functionality at emulsion interfaces. A review. Food Hydrocolloids, 24, 259–265.
Schmid, F. X. (1989). Spectra methods of characterizing protein conformation and conformational changes. In Creighton (Ed.), Protein structure: a practical approach (pp. 251–285). New York: Springer.
Speroni, F., Beaumal, V., de Lamballerie, M., Anton, M., Anon, M. C., & Puppo, M. C. (2009). Gelation of soybean proteins induced by sequential high-pressure and thermal treatments. Food Hydrocolloids, 23, 1433–1442.
Tan, S. H., Mailer, R. J., Blanchard, C. L., & Agboola, S. O. (2011). Canola proteins for human consumption: extraction, profile, and functional properties. Journal of Food Science, 76, R16–R28.
Tang, C. H., & Ma, C. Y. (2009). Effect of high pressure treatment on aggregation and structural properties of soy protein isolate. LWT−Food. Science and Technology, 42, 606–611.
Van der Plancken, I., Van Loey, A., & Hendrickx, M. E. (2007). Foaming properties of egg white proteins affected by heat or high pressure treatment. Journal of Food Engineering, 78, 1410–1426.
Vioque, J., Sanchez-Vioque, R., Clemente, A., Pedroche, J., & Millan, F. (2000). Partially hydrolyzed rapeseed protein isolates with improved functional properties. Journal of the American Oil Chemists’ Society, 77, 447–450.
Wang, C. H., & Damodaran, S. (1990). Thermal gelation of globular proteins: weight-average molecular weight dependence of gel strength. Journal of Agricultural and Food Chemistry, 38, 1157–1164.
Wang, X. S., Tang, C. H., Li, B. S., Yang, X. Q., Li, L., & Ma, C. Y. (2008). Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocolloids, 22, 560–567.
Wheeler, E. L., & Ferrel, R. E. (1971). A method for phytic acid determination in wheat and wheat fractions. Cereal Chemistry, 48, 312–320.
Whitmore, L., & Wallace, B. A. (2004). DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Research, 32, W668–W673.
Whitmore, L., & Wallace, B. A. (2008). Protein secondary structure analysis from circular dichroism spectroscopy: methods and reference databases. Biopolymers, 89, 392–400.
Wu, J., & Muir, A. D. (2008). Comparative structural, emulsifying, and biological properties of 2 major canola proteins, cruciferin and napin. Journal of Food Science, 73, C210–C216.
Wu, W. U., Hettiarachchy, N. S., & Qi, M. (1998). Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. Journal of the American Oil Chemists’ Society, 75, 845–850.
Yin, S. W., Tang, C. H., Wen, Q. B., Yang, X. Q., & Li, L. (2008). Functional properties and in vitro trypsin digestibility of red kidney bean (Phaseolus vulgaris L.) protein isolate: Effect of high-pressure treatment. Food Chemistry, 110, 938–945.
Yoshie-Stark, Y., Wada, Y., Schott, M., & Wäsche, A. (2006). Functional and bioactive properties of rapeseed protein concentrates and sensory analysis of food application with rapeseed protein concentrates. LWT- Food Science and Technology, 39, 503–512.
Yoshie-Stark, Y., Wada, Y., & Wäsche, A. (2008). Chemical composition, functional properties, and bioactivities of rapeseed protein isolates. Food Chemistry, 107, 32–39.
Zhang, H., Li, L., Tatsumi, E., & Kotwal, S. (2003). Influence of high pressure on conformational changes of soybean glycinin. Innovative Food Science & Emerging Technologies, 4, 269–275.
Acknowledgments
Funding for this work was provided through the National Key Technology Research and Development Program of Jiangsu Province, China (project no. SBE201130495). The research program of Dr. R.E. Aluko is funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) through a Discovery Grant.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, R., He, HY., Chao, D. et al. Effects of High Pressure and Heat Treatments on Physicochemical and Gelation Properties of Rapeseed Protein Isolate. Food Bioprocess Technol 7, 1344–1353 (2014). https://doi.org/10.1007/s11947-013-1139-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1139-z