Abstract
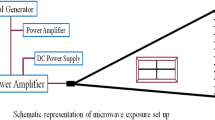
The object of this study is to investigate the effects of 50-GHz microwave radiation on the brain of Wistar rats. Male rats of the Wistar strain were used in the study. Animals of 60-day age were divided into two groups—group 1, sham-exposed, and group 2, experimental (microwave-exposed). The rats were housed in a temperature-controlled room (25 °C) with constant humidity (40–50%) and received food and water ad libitum. During exposure, rats were placed in Plexiglas cages with drilled ventilation holes and kept in an anechoic chamber. The animals were exposed for 2 h a day for 45 days continuously at a power level of 0.86 μW/cm2 with nominal specific absorption rate 8.0 × 10−4 w/kg. After the exposure period, the rats were killed and homogenized, and protein kinase C (PKC), DNA double-strand break, and antioxidant enzyme activity [superoxides dismutase (SOD), catalase, and glutathione peroxidase (GPx)] were estimated in the whole brain. Result shows that the chronic exposure to these radiations causes DNA double-strand break (head and tail length, intensity and tail migration) and a significant decrease in GPx and SOD activity (p = <0.05) in brain cells, whereas catalase activity shows significant increase in the exposed group of brain samples as compared with control (p = <0.001). In addition to these, PKC decreased significantly in whole brain and hippocampus (p < 0.05). All data are expressed as mean ± standard deviation. We conclude that these radiations can have a significant effect on the whole brain.





Similar content being viewed by others
References
Stuchley, M. A. (1988). Biological effects of radiofrequency fields. In M. H. Repacholi (Ed.), Non-Ionizing Radiations, Physical characterization, Biological effects and Health Hazard Assessment. Proceeding for the International Non-Ionizing Radiation Workshop. Melbourne, pp. 197–217.
Kunjilwar, K. K., & Behari, J. (1993). Effect of amplitude-modulated radio frequency radiation on cholinergic system of developing rats. Brain Research, 601, 321–324. doi:10.1016/0006-8993(93)91729-C.
Paulraj, R., & Behari, J. (2004). Radiofrequency radiation effect on protein kinase C activity in rats brain. Mutation Research, 585, 127–131. doi:10.1016/S0027-5107(03)00113-1.
Harvey, C., & French, P. W. (2000). Effects on protein kinase C and gene expression in a human mast cell line, HMC-1, following microwave exposure. Cell Biology International, 23, 739–748. doi:10.1006/cbir.1999.0436.
Nishizuka, Y. (1986). Studies and perspectives of protein kinase C. Science, 233, 305–312. doi:10.1126/science.3014651.
Ohkusu, K., Isobe, K., Hidaka, H., & Nakashima, I. (1986). Elucidation of the protein kinase C-dependent apoptosis pathway in distinct of T lymphocytes in MRL-lpr/lpr mice. European Journal of Immunology, 25, 3180–3186. doi:10.1002/eji.1830251129.
Suzuki, T. (1994). Protein kinase involved in the expression of long-term potentiation. The International Journal of Biochemistry, 26, 735–744. doi:10.1016/0020-711X(94)90102-3.
Castagna, M., Takai, Y., Kaibuchi, K., Sano, K., Kikkawa, U., & Nishizuka, Y. (1982). Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumorpromoting phorbol esters. The Journal of Biological Chemistry, 78, 47–51.
Hunter, T., Ling, N., & Cooper, J. A. (1984). Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature, 311, 480–483. doi:10.1038/311480a0.
Niedel, J. E., Kuhn, L. J., & Vandenbark, G. R. (1983). Phorbol diester receptor copurifies with protein kinase C. Proceedings of the National Academy of Sciences of the United States of America, 80, 36–40. doi:10.1073/pnas.80.1.36.
Mahfouz, R., Sharma, R., Lackner, J., Aziz, A., & Agarwal, A. (2008). Evaluation of chemiluminescence and flow cytometry as tools in assessing production of hydrogen peroxide and superoxide anion in human spermatozoa. Fertility and Sterility, in press. doi:10.1016/j.fertnstert.2008.05.087.
Behari, J., Kunjilwar, K. K., & Pyne, S. (1998). Interaction of low level modulated RF radiation with Na+–K+–ATPase. Bioelectrochemistry and Bioenergetics, 47, 247–252. doi:10.1016/S0302-4598(98)00195-0.
Paulraj, R., Behari, J., & Rao, A. R. (1999). Effect of 112 MHz amplitude modulated radiation on calcium ion efflux and ODC activity in chronically exposed rat brain. Indian Journal of Biochemistry & Biophysics, 36, 337–340.
Paulraj, R., & Behari, J. (2002). The effect of low level continuous 2.45 GHz wave on brain enzymes of developing rat brain. Electromagnetic Biology and Medicine, 21, 231–241. doi:10.1081/JBC-120015993.
Sarkar, S., Ali, S., & Behari, J. (1994). Effect of low power microwave on the mouse genome: a direct DNA analysis. Mutation Research, 320, 141–147. doi:10.1016/0165-1218(94)90066-3.
Paulraj, R., & Behari, J. (2006). Single strand DNA breaks in rat brain cells exposed to microwave radiation. Mutation Research, 596, 76–80. doi:10.1016/j.mrfmmm.2005.12.006.
Behari, J., & Kesari, K. K. (2006). Effects of microwave radiations on reproductive system of male rats. Embryo Talk, 1, 81–85.
Lai, H., & Singh, N. P. (1996). Double strand breaks in rats brain cells after acute exposure to radio frequency electromagnetic radiation. International Journal of Radiation Biology, 69, 513–521. doi:10.1080/095530096145814.
Singh, N. P., & Lai, H. (1998). 60-Hz magnetic field exposure induces DNA crosslinks in rat brain cells. Mutation Research, 400, 313–320. doi:10.1016/S0027-5107(98)00017-7.
Altman, S. A., Zastawny, T. H., Randers-Eichhorn, L., Cacciuttolo, M. A., Akman, S. A., & Dizdaroglu, M. (1995). Formation of DNAprotein cross-links in cultured mammalian cells upon treatment with iron ions. Free Radical Biology & Medicine, 19, 897–902. doi:10.1016/0891-5849(95)00095-F.
Lloyd, D. R., Phillips, D. W., & Carmichael, P. L. (1997). Generation of putative intrastrand cross-links and strand breaks by transition metal ion-mediated oxygen radiacl ttack. Chemical Research in Toxicology, 10, 393–400. doi:10.1021/tx960158q.
Ivancsits, S., Diem, E., Pilger, A., Rudiger, H. W., & Jahn, O. (2002). Induction of DNA strand breaks by intermittent exposure to extremely-low-frequency electromagnetic fields in human diploid fibroblasts. Mutation Research, 519, 1–13.
Ivancsits, S., Diem, E., Jahn, O., & Rudiger, H. W. (2003a). Intermittent extremely low frequency electromagnetic fields cause DNA damage in a dose-dependent way. International Archives of Occupational and Environmental Health, 76, 431–436. doi:10.1007/s00420-003-0446-5.
Ivancsits, S., Diem, E., Jahn, O., & Rudiger, H. W. (2003b). Age-related effects on induction of DNA strand breaks by intermittent exposure to electromagnetic fields. Mechanism of Ageing and Development, 124, 847–850.
Suzuki, T. (1994). Protein kinase involved in the expression of long-term potentiation. The International Journal of Biochemistry, 26, 735–744.
Sakuma, N., Komatsubara, Y., Takeda, H., Hirose, H., Sekijima, M., Nojima, T., & Miyakoshi, J. (2006). DNA strand breaks are not induced in human cells exposed to 2.1425 GHz band CW and W-CDMA modulated radiofrequency fields allocated to mobile radio base stations. Bioelectromagnetics, 27, 51–57. doi:10.1002/bem.20179.
Vijayalaxmi, , & Obe, G. (2004). Controversial cytogenetic observations in mammalian somatic cells exposed to radiofrequency radiation. Radiation Research, 162, 481–496.
Lowry, O. H., Resebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with folin-phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Singh, N. (2003). In R. Blumenthal (Ed.), Apoptosis by DNA diffusion assay, methods in molecular medicine (chemosensitivity). Totowas: Humana.
Tice, R. R., Agurell, E., Anderson, D., Burlinson, B., Hartmann, A., Kobayashi, H., et al. (2000). Single cell gel/comet assay; guidelines for in vitro and in vivo genetic toxicology testing. Environmental and Molecular Mutagenesis, 35, 206–221. doi:10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J.
Rotilio, G. (1972). Effect of hydrogen peroxide on dismutase and catalase activity in rat liver. Biochemichistry, 11, 2187–2189.
Alvarez, J. G., Touchstone, C. J., Blasco, L., & Storey, B. T. (1987). Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. SOD as major enzyme protectant against oxygen toxicity. Journal of Andrology, 8, 33–89.
Condell, R. A., & Tappel, A. L. (1993). Evidence for suitability of glutathione peroxidase as a protective enzyme: studies of oxidative damage, restoration and proteolysis. Archives of Biochemistry and Biophysics, 223, 407. doi:10.1016/0003-9861(83)90604-5.
Reiter, R. J. (1997). Melatonin aspects of exposure to low frequency electric and magnetic fields. Advances in electromagnetic fields in living systems (vol. 2, (pp. 1–27)). New York: Plenum.
Jones, D. P., Eklow, L., Thor, H., & Orrenius, S. (1981). Metabolism of hydrogen peroxide in isolated hepatocytes: relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated H2O2 Arch. Biochemistry & Biophysics, 210, 505–516. doi:10.1016/0003-9861(81)90215-0.
Chen, G., Upham, B. L., & Sun, W. (2000). Effect of electromagnetic field exposure on chemically induced differentiation of friend erythroleukemia cells. Environmental Health Perspectives, 108, 967–972. doi:10.2307/3435056.
Brydon, L., Petit, L., Delagrange, P., Strosberg, A. D., & Jockers, R. (2000). Functional expression of MT2 (Mel 1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology, 142, 4264–4271. doi:10.1210/en.142.10.4264.
Suzuki, Y. J., Forman, H. J., & Sevanian, A. (1997). Oxidants as stimulators of signal transduction. Free Radical Biology & Medicine, 22, 269–285. doi:10.1016/S0891-5849(96)00275-4.
Rodnight, R., Grower, H., Martinez-Millan, L., & DeSouza, D. (1982). Molecular aspects of neural transmission, learning and memory. In R. Caputto, & C. Ajmone-Marsan (Eds.), IBRO, vol. 10 (p. 125). New York: Raven.
Butler, A. P., Mar, P. K., McDonald, F. F., & Ramsay, R. L. (1991). Involvement of protein kinase C in the regulation of ornithine decarboxylase mRNA by phorbol esters in rat hepatoma cells. Experimental Cell Research, 194, 56–61. doi:10.1016/0014-4827(91)90129-I.
Byus, C. V., Kartun, K., Pieper, S. E., & Adey, W. R. (1988). Increased ornithine decarboxylase activity in cultured cells exposed to low energy modulated microwave fields and phorbol ester tumor promoters. Cancer Research, 48, 4222–4226.
Lai, H., & Singh, N. P. (1995). Acute low-intensity microwave exposure increases DNA single-strand breaks in rat brain cells. Bioelectromagnetics, 16, 207–210. doi:10.1002/bem.2250160309.
Lai, H., & Singh, N. P. (2005). Interaction of microwaves and a temporally incoherent magnetic field on single and double DNA strand breaks in rat brain cells. Electromagnetic Biology and Medicine, 24, 23–29. doi:10.1081/JBC-200055046.
Lai, H., & Singh, N. P. (1997a). Acute exposure to a 60-Hz magnetic field increases DNA strand breaks in rat brain cells. Bioelectromagnetics, 18, 156–165. doi:10.1002/(SICI)1521-186X(1997)18:2<156::AID-BEM8>3.0.CO;2-1.
Nikolova, T., Czyz, J., Rolletschek, A., Blyszczuk, P., Fuchs, J., Jovtchev, G., et al. (2005). Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. ASEB J, 19(12), 1686–1688.
Aitken, R. J., Bennetts, L. E., & Sawyer, D. (2005). Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. International Journal of Andrology, 28, 171–179. doi:10.1111/j.1365-2605.2005.00531.x.
Diem, E., Schwarz, C., Adlkofer, F., Jahn, O., & Rudiger, H. (200). Non-thermal DNA breakage by mobile-phone radiation. (1800). MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutation Research, 583(2), 178–183.
Agarwal, A., Deepinder, F., & Sharma, R. K. (2007). Effect of cell phone usage on semen analysis in men attending infertility clinic: An observational study. Fertility and Sterility doi:10.1016/j.fertnstert.2007.01.166.
Agarwal, A., Deepinder, F., Sharma, R. K., Ranga, G., & Li, J. (2008). Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertility and Sterility, 89(1), 124–128. doi:10.1016/j.fertnstert.2007.01.166.
Acknowledgment
Authors are thankful to Council for Scientific and Industrial Research (CSIR), New Delhi, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kesari, K.K., Behari, J. Fifty-gigahertz Microwave Exposure Effect of Radiations on Rat Brain. Appl Biochem Biotechnol 158, 126–139 (2009). https://doi.org/10.1007/s12010-008-8469-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8469-8