Abstract
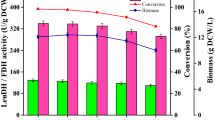
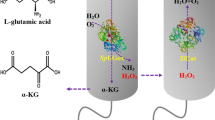
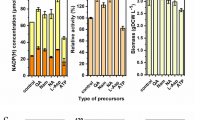
d-amino acid oxidase (DAAO) is widely used in the industrial preparation of l-amino acids, and cultivating Escherichia coli (E. coli) expressing DAAO for the biosynthesis of l-phosphinothricin (l-PPT) is very attractive. At present, the biomass production of DAAO by fermentation is still limited in large-scale industrial applications because the expression of DAAO during the fermentation process inhibits the growth of host cells, which limits higher cell density. In this study, the factors that inhibit the growth of bacterial cells during a 5-L fed-batch fermentation process were explored, and the fermentation process was optimized by co-expressing catalase (CAT), by balancing the biomass and the enzyme activity, and by adding exogenous d-alanine (d-Ala) to relieve the limitation of DAAO on the cells and optimize fermentation. Under optimal conditions, the DO-STAT feeding mode with DO controlled at 30% ± 5% and the addition of 27.5 g/L lactose mixed with 2 g/L d-Ala during induction at 28 °C resulted in the production of 26.03 g dry cell weight (DCW)/L biomass and 390.0 U/g DCW specific activity of DAAO; an increase of 78% and 84%, respectively, compared with the initial fermentation conditions. The fermentation strategy was successfully scale-up to a 5000-L fermenter.







Similar content being viewed by others
Data Availability
The authors declare that the data and materials are transparent.
Code Availability
All data, models, and code generated or used during the study appear in the submitted article.
References
Han, H. J., Zhu, B., Fu, X. Y., You, S. H., Wang, B., Li, Z. J., Zhao, W., Peng, R. H., & Yao, Q. H. (2015). Overexpression of d-amino acid oxidase from Bradyrhizobium japonicum, enhances resistance to glyphosate in Arabidopsis thaliana. Plant Cell Reports, 34(12), 2043–2051. https://doi.org/10.1007/s00299-015-1850-5.
Han, L., Zhao, Y. K., Cui, S., & Liang, B. (2018). Redesigning of microbial cell surface and its application to whole-cell biocatalysis and biosensors. Applied Biochemistry and Biotechnology, 185(2), 396–418. https://doi.org/10.1007/s12010-017-2662-6.
Pollegioni, L., & Molla, G. (2011). New biotech applications from evolved d-amino acid oxidases. Trends in Biotechnology, 29(6), 276–283. https://doi.org/10.1016/j.tibtech.2011.01.010.
Takahashi, S., Abe, K., & Kera, Y. (2015). Bacterial d-amino acid oxidases: Recent findings and future perspectives. Bioengineered, 6(4), 237–241. https://doi.org/10.1080/21655979.2015.1052917.
Xue, Y. P., Cao, C. H., & Zheng, Y. G. (2018). Enzymatic asymmetric synthesis of chiral amino acids. Chemical Society Reviews, 47(4), 1516–1561. https://doi.org/10.1039/C7CS00253J.
Fotheringham, I., Archer, I., Carr, R., Speight, R., & Turner, N. J. (2006). Preparative deracemization of unnatural amino acids. Biochemical Society Transactions, 34(2), 287–290. https://doi.org/10.1042/BST0340287.
Pilone, M. S., & Pollegioni, L. (2009). Enzymes, d-amino acid oxidases. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology (pp. 1–13).
Chen, Y., Goldberg, S. L., Hanson, R. L., Parker, W. L., Gill, I., Tully, T. P., Montana, M. A., Goswami, A., & Patel, R. N. (2011). Enzymatic preparation of an (S)-amino acid from a racemic amino acid. Organic Process Research & Development, 15(1), 241–248. https://doi.org/10.1021/op1001534.
Trost, E. M., & Fischer, L. (2002). Minimization of by-product formation during d-amino acid oxidase catalyzed racemate resolution of d/l-amino acids. Journal of Molecular Catalysis B: Enzymatic, 19, 189–195. https://doi.org/10.1016/S1381-1177(02)00166-2.
Bianchi, D., Bortolo, R., Golini, P., & Cesti, P. (1998). Enzymatic transformation of cephalosporin c to 7-aca by Simultaneous Action of Immobilized d-amino acid oxidase and glutaryl-7-ACA acylase. Applied Biochemistry and Biotechnology, 73(2-3), 257–268. https://doi.org/10.1007/BF02785660.
Hardianto, D., Royani, J. I., & Safarrida, A. (2016). Cephalosporin C acylase from microbes for one-step enzymatic transformation of cephalosporin C to 7-aminocephalosporanic acid. Journal of Pure and Applied Microbiology, 10(4), 2495–2499. https://doi.org/10.22207/jpam.10.4.03.
Ma, X. Q., Deng, S. W., Su, E. Z., & Wei, D. Z. (2015). One-pot enzymatic production of deacetyl-7-aminocephalosporanic acid from cephalosporin C via immobilized cephalosporin C acylase and deacetylase. Biochemical Engineering Journal, 95, 1–8. https://doi.org/10.1016/j.bej.2014.11.015.
Duke, S. O. (2018). The history and current status of glyphosate. Pest Management Science, 74(5), 1027–1034. https://doi.org/10.1002/ps.4652.
Freudl, R. (2018). Signal peptides for recombinant protein secretion in bacterial expression systems. Microbial Cell Factories, 17(1), 52. https://doi.org/10.1186/s12934-018-0901-3.
Kang, X. M., Cai, X., Liu, Z. Q., & Zheng, Y. G. (2019). Identification and characterization of an amidase from Leclercia adecarboxylata for efficient biosynthesis of l-phosphinothricin. Bioresource Technology, 289, 121658. https://doi.org/10.1016/j.biortech.2019.121658.
Chien, L. J., Wu, J. M., Kuan, I. C., & Lee, C. K. (2004). Coexpression of Vitreoscilla hemoglobin reduces the toxic effect of expression of d-amino acid oxidase in E. coli. Biotechnology Progress, 20(5), 1359–1365. https://doi.org/10.1021/bp0498589.
Kim, S. J., Kim, N. J., Shin, C. H., & Kim, C. W. (2008). Optimization of culture condition for the production of d-amino acid oxidase in a recombinant Escherichia coli. Biotechnology and Bioprocess Engineering, 13(2), 144–149. https://doi.org/10.1007/s12257-008-0005-8.
Zheng, J. X., Yang, T. W., Zhou, J. P., Xu, M. J., Zhang, X., Rao, Z. M., & Yang, S. (2017). Efficient production of d-amino acid oxidase in Escherichia coli by a trade-off between its expression and biomass using N-terminal modification. Bioresource Technology, 243, 716–723. https://doi.org/10.1016/j.biortech.2017.07.007.
Kuru, E., Hughes, H. V., Brown, P. J., Hall, E., Tekkam, S., Cava, F., de Pedro, M. A., Brun, Y. V., & VanNieuwenhze, M. S. (2012). In situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent d-amino acids. Angewandte Chemie, 51(50), 12519–12523. https://doi.org/10.1002/ange.201206749.
Jang, S., & Imlay, J. A. (2007). Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. Journal of Biological Chemistry, 282(2), 929–937. https://doi.org/10.1002/ange.201206749.
Kumar, S. R., & Imlay, J. A. (2013). How Escherichia coli tolerates profuse hydrogen peroxide formation by a catabolic pathway. Journal of Bacteriology, 195(20), 4569–4579. https://doi.org/10.1128/JB.00737-13.
Park, S., You, X., & Imlay, J. A. (2005). Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 102(26), 9317–9322. https://doi.org/10.1074/jbc.M607646200.
Xu, J. M., Zhang, K., Cao, H. T., Li, H., Cheng, F., Cao, C. H., Xue, Y. P., & Zheng, Y. G. (2020). Development of a biocatalytic cascade for synthesis of 2-oxo-4-(hydroxymethylphosphinyl) butyric acid in one pot. Biocatalysis and Biotransformation, 1–8. https://doi.org/10.1080/10242422.2020.1797697.
Cao, C. H., Gong, H., Dong, Y., Li, J. M., Cheng, F., Xue, Y. P., & Zheng, Y. G. (2020). Enzyme cascade for biocatalytic deracemization of d, l-phosphinothricin. Journal of Biotechnology, 325, 372–379. https://doi.org/10.1016/j.jbiotec.2020.09.024.
Cao, C. H., Cheng, F., Xue, Y. P., & Zheng, Y. G. (2020). Efficient synthesis of l-phosphinothricin using a novel aminoacylase mined from Stenotrophomonas maltophilia. Enzyme and Microbial Technology, 135, 109493. https://doi.org/10.1016/j.enzmictec.2019.109493.
Lv, S. Z., Guo, Y. X., Xue, Y. P., Xu, J. M., & Zheng, Y. G. (2019). Efficient separation of l-phosphinothricin from enzymatic reaction solution using cation-exchange resin. Separation Science and Technology, 55(4), 779–787. https://doi.org/10.1016/j.enzmictec.2019.109493.
Luo, H., Li, Q., Yu, H., & Shen, Z. (2004). Construction and application of fusion proteins of d-amino acid oxidase and glutaryl-7-aminocephalosporanic acid acylase for direct bioconversion of cephalosporin C to 7-aminocephalosporanic acid. Biotechnology Letters, 26(11), 939–945. https://doi.org/10.1023/B:bile.0000025907.33332.be.
Zhang, C. C., Zhang, S. S., Liu, W., Guo, T. T., Gu, R. X., & Kong, J. (2019). Potential application and bactericidal mechanism of lactic acid-hydrogen peroxide consortium. Applied Biochemistry and Biotechnology, 189(3), 822–833. https://doi.org/10.1007/s12010-019-03031-z.
Ju, S. S., Lin, L. L., Chien, H. R., & Hsu, W. H. (2000). Substitution of the critical methionine residues in Trigonopsis variabilis d-amino acid oxidase with leucine enhances its resistance to hydrogen peroxide. FEMS Microbiology Letters, 186(2), 215–219. https://doi.org/10.1111/j.1574-6968.2000.tb09107.x.
Alfonso-Prieto, M., Vidossich, P., & Rovira, C. (2012). The reaction mechanisms of heme catalases: an atomistic view by ab initio molecular dynamics. Archives of Biochemistry and Biophysics, 525(2), 121–130. https://doi.org/10.1016/j.abb.2012.04.004.
Gebicka, L., & Krych-Madej, J. (2019). The role of catalases in the prevention/promotion of oxidative stress. Journal of Inorganic Biochemistry, 197, 110699. https://doi.org/10.1016/j.jinorgbio.2019.110699.
Young, G. B., Jack, D. L., Smith, D. W., & Saier Jr., M. (1999). The amino acid/auxin: proton symport permease family. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1415(2), 306–322. https://doi.org/10.1016/S0005-2736(98)00196-5.
Romano, D., Molla, G., Pollegioni, L., & Marinelli, F. (2009). Optimization of human d-amino acid oxidase expression in Escherichia coli. Protein Expression and Purification, 68(1), 72–78. https://doi.org/10.1016/j.pep.2009.05.013.
Deng, S. W., Su, E. Z., Ma, X. Q., Yang, S., & Wei, D. (2014). High-level soluble and functional expression of Trigonopsis variabilis d-amino acid oxidase in Escherichia coli. Bioprocess and Biosystems Engineering, 37(8), 1517–1526. https://doi.org/10.1007/s00449-013-1123-z.
Kim, S. J., Park, H. W., Shin, C. H., & Kim, C. W. (2014). Establishment of a cryopreservation method for the industrial Use of d-amino acid oxidase-overexpressing Escherichia coli. Bioscience, Biotechnology, and Biochemistry, 73(2), 299–303. https://doi.org/10.1271/bbb.80507.
Funding
This work was financially supported by the National Key R & D Program of China (2018YFA0901400).
Author information
Authors and Affiliations
Contributions
Jian-Miao Xu, Hui-Ting Cao, and Kai Zhang made substantial contributions to the design of the work, performed the most analysis, experiments, and wrote the manuscript. Ming Wang, Bao-Jian Ma, and Liu-Yu Wang participate in the experiment. Jian-Miao Xu, Feng Cheng, Ya-Ping Xue, and Yu-Guo Zheng revised it critically for important intellectual content and approved the version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, JM., Cao, HT., Wang, M. et al. Development of a Combination Fermentation Strategy to Simultaneously Increase Biomass and Enzyme Activity of d-amino Acid Oxidase Expressed in Escherichia coli. Appl Biochem Biotechnol 193, 2029–2042 (2021). https://doi.org/10.1007/s12010-021-03519-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03519-7