Abstract
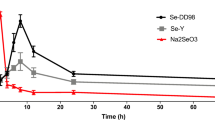
For the first time, bioavailability, pharmacokinetics, and biotransformation of selenium-enriched yeast (SeY) and sodium selenite (Na2SeO3) in rats were systemically compared by analyzing free selenomethionine (SeMet), total SeMet, and selenium (Se). After SeY and Na2SeO3 were orally administered to rats at a dose of 100 μg Se/kg, plasma free SeMet, total SeMet, and Se at various time points were determined by ultra-performance liquid chromatography-tandem mass spectrometry. Based on Se and total SeMet, the relative bioavailability values of SeY compared with Na2SeO3 were 144% and 272%, respectively. For the rats treated with SeY, 0.73–2.68% of total Se was biotransformed to free SeMet, 14.3–20.4% to SeMet-proteins and albumin-bound SeMet, and 75.9–82.3% to selenoproteins in plasma. SeY had higher bioavailability than Na2SeO3 based on Se and total SeMet levels. Plasma SeMet was the optimal biomarker of SeY status in vivo.

Similar content being viewed by others
References
Suzuki KT (2005) Metabolomics of selenium: Se metabolites based on speciation studies. J Health Sci 51(2):107–114
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1):25–54. https://doi.org/10.1039/c3mt00185g
Combs GF Jr, Clark LC, Turnbull BW (2001) An analysis of cancer prevention by selenium. Biofactors 14(1-4):153–159
Encinar JR, Schaumloffel D, Ogra Y, Lobinski R (2004) Determination of selenomethionine and selenocysteine in human serum using speciated isotope dilution-capillary HPLC-inductively coupled plasma collision cell mass spectrometry. Anal Chem 76(22):6635–6642. https://doi.org/10.1021/ac049280h
Bier K, Vacchina V, Szpunar J, Bertinc G, Lobinnski R (2008) Simultaneous derivatization of selenocysteine and selenomethionine in animal blood prior to their specific determination by 2D size-exclusion ion-pairing reversed-phase HPLC-ICP MS. J Anal At Spectrom 23:508–513
Zhang SQ, Zhang HB, Zhang Y (2018) Quantification of selenomethionine in plasma using UPLC-MS/MS after the oral administration of selenium-enriched yeast to rats. Food Chem 241:1–6. https://doi.org/10.1016/j.foodchem.2017.08.068
Hoefig CS, Renko K, Kohrle J, Birringer M, Schomburg L (2011) Comparison of different selenocompounds with respect to nutritional value vs. toxicity using liver cells in culture. J Nutr Biochem 22(10):945–955. https://doi.org/10.1016/j.jnutbio.2010.08.006
Rayman MP, Infante HG, Sargent M (2008) Food-chain selenium and human health: spotlight on speciation. Br J Nutr 100(2):238–253. https://doi.org/10.1017/S0007114508922522
European Food Safety Authority (2008) Selenium-enriched yeast as source for selenium added for nutritional purposes in foods for particular nutritional uses and foods (including food supplements) for the general population - scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food. EFSA J 6(7):766. https://doi.org/10.2903/j.efsa.2008.766
Yoshida M, Fukunaga K, Tsuchita H, Yasumoto K (1999) An evaluation of the bioavailability of selenium in high-selenium yeast. J Nutr Sci Vitaminol (Tokyo) 45(1):119–128
Takahashi K, Suzuki N, Ogra Y (2017) Bioavailability comparison of nine bioselenocompounds in vitro and in vivo. Int J Mol Sci 18(3):506. https://doi.org/10.3390/ijms18030506
Bogye G, Alfthan G, Machay T (1998) Bioavailability of enteral yeast-selenium in preterm infants. Biol Trace Elem Res 65(2):143–151
Liu X, Piao J, Huang Z, Zhang SQ, Li W, Tian Y, Yang X (2014) Determination of 16 selected trace elements in children plasma from china economical developed rural areas using high resolution magnetic sector inductively coupled mass spectrometry. J Anal Methods Chem 2014:975820. https://doi.org/10.1155/2014/975820
Suzuki KT, Ogra Y (2002) Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched Se. Food Addit Contam 19(10):974–983. https://doi.org/10.1080/02652030210153578
Nyman DW, Suzanne Stratton M, Kopplin MJ, Dalkin BL, Nagle RB, Jay Gandolfi A (2004) Selenium and selenomethionine levels in prostate cancer patients. Cancer Detect Prev 28(1):8–16. https://doi.org/10.1016/j.cdp.2003.11.002
Dumont E, Vanhaecke F, Cornelis R (2006) Selenium speciation from food source to metabolites: a critical review. Anal Bioanal Chem 385(7):1304–1323. https://doi.org/10.1007/s00216-006-0529-8
Reyes LH, Encinar JR, Marchante-Gayon JM, Alonso JI, Sanz-Medel A (2006) Selenium bioaccessibility assessment in selenized yeast after “in vitro” gastrointestinal digestion using two-dimensional chromatography and mass spectrometry. J Chromatogr A 1110(1-2):108–116. https://doi.org/10.1016/j.chroma.2006.01.088
Funding
The work was partly supported by the Hubei Provincial Natural Science Foundation of China (2018CFB612).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Animal experiments were adhered to the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, eighth edition in 2011) and were approved by our Institutional Animal Care and Use Committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, SQ., Shen, S. & Zhang, Y. Comparison of Bioavailability, Pharmacokinetics, and Biotransformation of Selenium-Enriched Yeast and Sodium Selenite in Rats Using Plasma Selenium and Selenomethionine. Biol Trace Elem Res 196, 512–516 (2020). https://doi.org/10.1007/s12011-019-01935-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01935-9