Abstract
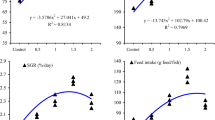
The present investigation aimed to evaluate the influence of copper nanoparticles (Cu-NPs) on the growth, immunity, and oxidation resistance of common carp (3.02 ± 0.01 g, initial mean weight ± S.E.). Five groups of fish fed diets with Cu-NPs at 0, 0.5, 1, 2, and 4 mg/kg for 8 weeks. The results suggested that Cu-NPs in diets increased the growth performance and reduced FCR with linear and quadratic model (P < 0.05). Also, common carp fed Cu-NPs showed increased carcass protein, lipid, and ash contents in a dose-dependent manner (P < 0.05). The Cu accumulation in the carcass, liver, muscle, and gills increased by Cu-NPs and showed the maximum at 4 mg Cu-NPs/kg (P < 0.05). No significant alterations were found in the blood variables due to Cu-NP supplementation except for the Hb, RBCs, total protein, albumin, and globulin levels which showed the highest level in 2 mg/kg (P < 0.05). IgM level, phagocytic, lysozyme, SOD, CAT, and GPX activities were boosted by Cu-NPs with decreased malondialdehyde (MDA) content (P < 0.05). Based on regression analysis, the requirement of dietary Cu-NPs for common carp was estimated to be 2.19 to 2.91 mg/kg diet.


Similar content being viewed by others
References
Moss AS, Ishikawa M, Koshio S, Yokoyama S, Dawood MAO (2019) Effects of different levels of marine snail shells in the diets of juvenile Kuruma shrimps Marsupenaeus japonicus as a source of calcium. N Am J Aquac 81(1):55–66
Wang W, Ishikawa M, Koshio S, Yokoyama S, Dawood MAO, Hossain MS, Zaineldin AI (2019) Interactive effects of dietary astaxanthin and cholesterol on the growth, pigmentation, fatty acid analysis, immune response and stress resistance of kuruma shrimp (Marsupenaeus japonicus). Aquac Nutr 25(4):946–958
Ahmadifar E, Sadegh TH, Dawood MAO, Dadar M, Sheikhzadeh N (2020) The effects of dietary Pediococcus pentosaceus on growth performance, hemato-immunological parameters and digestive enzyme activities of common carp (Cyprinus carpio). Aquaculture 516:734656
Ahmadifar E, Moghadam MS, Dawood MAO, Hoseinifar SH (2019) Lactobacillus fermentum and/or ferulic acid improved the immune responses, antioxidative defence and resistance against Aeromonas hydrophila in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol 94:916–923
Satoh S (1991) Common carp, Cyprinus carpio. In: Handbook of Nutrient Requirements of Finfish. CRC Press 2017, pp 55–68
Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151(1–4):185–207
Aliko V, Hajdaraj G, Caci A, Faggio C (2015) Copper induced lysosomal membrane destabilisation in haemolymph cells of Mediterranean green crab (Carcinus aestuarii, Nardo, 1847) from the Narta lagoon (Albania). Braz Arch Biol Technol 58(5):750–756
Vajargah MF, Yalsuyi AM, Sattari M, Prokić MD, Faggio C (2020) Effects of copper oxide nanoparticles (CuO-NPs) on parturition time, survival rate and reproductive success of guppy fish, Poecilia reticulata. J Clust Sci 31:499–506
O'dell B, Campbell B (1970) Trace elements: metabolism and metabolic function. Comprehensive biochemistry 21:179–266.
Bonham M, O'Connor JM, Hannigan BM, Strain JJ (2002) The immune system as a physiological indicator of marginal copper status? Br J Nutr 87(5):393–403
Lin Y-H, Shih C-C, Kent M, Shiau S-Y (2010) Dietary copper requirement reevaluation for juvenile grouper Epinephelus malabaricus, with an organic copper source. Aquaculture 310(1–2):173–177
Tan XY, Luo Z, Liu X, Xie CX (2011) Dietary copper requirement of juvenile yellow catfish Pelteobagrus fulvidraco. Aquac Nutr 17(2):170–176
Muralisankar T, Bhavan PS, Srinivasan V, Radhakrishnan S, Seenivasan C, Manickam N (2015) Effects of dietary copper on the growth physiology and biochemistry of the freshwater prawn Macrobrachium rosenbergii post larvae. Int J Pure Appl Biosci 3:509–518
Forouhar Vajargah M, Mohamadi Yalsuyi A, Hedayati A, Faggio C (2018) Histopathological lesions and toxicity in common carp (Cyprinus carpio L. 1758) induced by copper nanoparticles. Microsc Res Tech 81(7):724–729
Nordberg GF, Fowler BA, Nordberg M (2015) Toxicology of metals: overview, definitions, concepts, and trends. In: Handbook on the Toxicology of Metals. Elsevier, pp 1–12
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB (2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 126:104–115
Moyson S, Liew HJ, Fazio A, Van Dooren N, Delcroix A, Faggio C, Blust R, De Boeck G (2016) Kidney activity increases in copper exposed goldfish (Carassius auratus auratus). Comp Biochem Physiol C Toxicol Pharmacol 190:32–37
El Basuini MF, El-Hais AM, Dawood MAO, Abou-Zeid AE-S, El-Damrawy SZ, Khalafalla MMELS, Koshio S, Ishikawa M, Dossou S (2016) Effect of different levels of dietary copper nanoparticles and copper sulfate on growth performance, blood biochemical profiles, antioxidant status and immune response of red sea bream (Pagrus major). Aquaculture 455:32–40
Chen K, Yamamoto FY, Gatlin DM III (2020) Effects of inorganic and organic dietary copper supplementation on growth performance and tissue composition for juvenile red drum (Sciaenops ocellatus L.). Aquac Nutr. https://doi.org/10.1111/anu.13041
Shaw BJ, Handy RD (2011) Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions. Environ Int 37(6):1083–1097
Dawood MAO, Zommara M, Eweedah NM, Helal AI (2020) The evaluation of growth performance, blood health, oxidative status and immune-related gene expression in Nile tilapia (Oreochromis niloticus) fed dietary nanoselenium spheres produced by lactic acid bacteria. Aquaculture 515:734571
Zahin N, Anwar R, Tewari D, Kabir MT, Sajid A, Mathew B, Uddin MS, Aleya L, Abdel-Daim MM (2019) Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-05211-0
El-Seedi HR, El-Shabasy RM, Khalifa SA, Saeed A, Shah A, Shah R, Iftikhar FJ, Abdel-Daim MM, Omri A, Hajrahand NH (2019) Metal nanoparticles fabricated by green chemistry using natural extracts: biosynthesis, mechanisms, and applications. RSC Adv 9(42):24539–24559
Abdel-Daim MM, Eissa IAM, Abdeen A, Abdel-Latif HMR, Ismail M, Dawood MAO, Hassan AM (2019) Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ Toxicol Pharmacol 69:44–50
Ates M, Dugo M, Demir V, Arslan Z, Tchounwou PJ (2014) Effect of copper oxide nanoparticles to sheepshead minnow (Cyprinodon variegatus) at different salinities. Dig J Nanomater Biostruct 9(1):369
Wang T, Long X, Cheng Y, Liu Z, Yan S (2015) A comparison effect of copper nanoparticles versus copper sulphate on juvenile Epinephelus coioides: growth parameters, digestive enzymes, body composition, and histology as biomarkers. Int J Genomics 2015:783021
Wang T, Long X, Liu Z, Cheng Y, Yan S (2015) Effect of copper nanoparticles and copper sulphate on oxidation stress, cell apoptosis and immune responses in the intestines of juvenile Epinephelus coioides. Fish Shellfish Immunol 44(2):674–682
Muralisankar T, Bhavan PS, Radhakrishnan S, Seenivasan C, Srinivasan V (2016) The effect of copper nanoparticles supplementation on freshwater prawn Macrobrachium rosenbergii post larvae. J Trace Elem Med Biol 34:39–49
Wang H, Zhu H, Wang X, Li E, Du Z, Qin J, Chen L (2018) Comparison of copper bioavailability in copper-methionine, nano-copper oxide and copper sulfate additives in the diet of Russian sturgeon Acipenser gueldenstaedtii. Aquaculture 482:146–154
El Basuini MF, El-Hais AM, Dawood MAO, Abou-Zeid AE-S, EL-Damrawy SZ, Khalafalla MME-S, Koshio S, Ishikawa M, Dossou S (2017) Effects of dietary copper nanoparticles and vitamin C supplementations on growth performance, immune response and stress resistance of red sea bream, Pagrus major. Aquac Nutr 23(6):1329–1340
Dawood MA, Shukry M, Zayed MM, Omar AA, Zaineldin AI, El Basuini MF (2019) Digestive enzymes, immunity and oxidative status of Nile tilapia (Oreochromis niloticus) reared in intensive conditions. Slov Vet Res 56(22-Suppl). https://doi.org/10.26873/SVR-747-2019
Uribe C, Folch H, Enríquez R, Moran G (2011) Innate and adaptive immunity in teleost fish: a review. Veterinarni Medicina 56(10):486–503
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55(1):373–399
AOAC, Association of Official Analytical Chemists (1998) Official methods of analysis of official analytical chemists international, 16th ed. Washington, DC.
Ajani E, Akpoilih BU (2012) Growth responses and ionic regulation in common carp (Cyprinus carpio) after chronic dietary copper exposure and recovery. J Environ Anal Toxicol 2(143):2161. https://doi.org/10.4172/2161-0525.1000143
Houston A (1990) Blood and circulation/methods for fish biology. NY.: Amer. Fish, Society
Doumas BT, Bayse DD, Carter RJ, Peters T, Schaffer R (1981) A candidate reference method for determination of total protein in serum. I. Development and validation. Clin Chem 27(10):1642–1650
Dumas BT, Biggs HG (1972) Standard methods of clinical chemistry. Ed., Academic Press, New York
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Heinegård D, Tiderström G (1973) Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta 43(3):305–310
Coulombe JJ, Favreau L (1963) A new simple semimicro method for colorimetric determination of urea. Clin Chem 9:102–108
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6(1):24–27
Ellis A, Stolen J, Fletcher T, Anderson D, Robertson B, Van Muiswinkel W (1990) Lysozyme assay in techniques in fish immunology. Technique in Fish Immunology
Cai W-q, Li S-f, Ma J-y (2004) Diseases resistance of Nile tilapia (Oreochromis niloticus), blue tilapia (Oreochromis aureus) and their hybrid (female Nile tilapia×male blue tilapia) to Aeromonas sobria. Aquaculture 229(1):79–87
Yossa R, Verdegem M (2015) Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture 437:344–350
Berntssen MH, Hylland K, Bonga SEW, Maage A (1999) Toxic levels of dietary copper in Atlantic salmon (Salmo salar L.) parr. Aquat Toxicol 46(2):87–99
Sun S, Qin J, Yu N, Ge X, Jiang H, Chen L (2013) Effect of dietary copper on the growth performance, non-specific immunity and resistance to Aeromonas hydrophila of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol 34(5):1195–1201
Minganti V, Drava G, De Pellegrini R, Siccardi C (2010) Trace elements in farmed and wild gilthead seabream, Sparus aurata. Mar Pollut Bull 60(11):2022–2025
Dorton KL, Engle T, Hamar D, Siciliano P, Yemm RS (2003) Effects of copper source and concentration on copper status and immune function in growing and finishing steers. Anim Feed Sci Technol 110(1–4):31–44
Engstad RE, Robertsen B, Frivold E (1992) Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol 2(4):287–297
Ji L, Sun G, Li J, Wang Y, Du Y, Li X, Liu Y (2017) Effect of dietary β-glucan on growth, survival and regulation of immune processes in rainbow trout (Oncorhynchus mykiss) infected by Aeromonas salmonicida. Fish Shellfish Immunol 64:56–67
Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35(12):1366–1375
Dawood MAO, Zommara M, Eweedah NM, Helal AI (2019) Synergistic effects of selenium nanoparticles and vitamin E on growth, immune-related gene expression, and regulation of antioxidant status of Nile tilapia (Oreochromis niloticus). Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01857-6
Moustafa EM, Dawood MAO, Assar DH, Omar AA, Elbialy ZI, Farrag FA, Shukry M, Zayed MM (2020) Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 515:734589
Dawood MAO, Zommara M, Eweedah NM, Helal AI, Aboel-Darag MA (2020) The potential role of nano-selenium and vitamin C on the performances of Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-07651-5
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 446:25–29
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dawood, M.A.O., Eweedah, N.M., Moustafa, E.M. et al. Copper Nanoparticles Mitigate the Growth, Immunity, and Oxidation Resistance in Common Carp (Cyprinus carpio). Biol Trace Elem Res 198, 283–292 (2020). https://doi.org/10.1007/s12011-020-02068-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02068-0