Abstract
The natural healing ability of tendon is limited, and it cannot restore the native structure and function of tendon injuries. Tendon-derived stem cells (TDSCs) are a new type of pluripotent stem cells with multi-directional differentiation potential and are expected to become a promising cell-seed for the treatment of tendon injuries in the future. In this review, we outline the latest advances in the culture and identification of TDSCs. In addition, the influencing factors on the differentiation of TDSCs are discussed. Moreover, we aim to discuss recent studies to enhance TDSCs treatment of injured tendons. Finally, we identify the limitations of the current understanding of TDSCs biology, the main challenges of using their use, and potential therapeutic strategies to inform cell-based tendon repair.
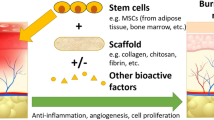
Graphical abstract



Similar content being viewed by others
Data Availability
Not applicable.
References
Holladay, C., Abbah, S. A., O'Dowd, C., Pandit, A., & Zeugolis, D. I. (2016). Preferential tendon stem cell response to growth factor supplementation. Journal of Tissue Engineering and Regenerative Medicine, 10(9), 783–798. https://doi.org/10.1002/term.1852.
Schneider, M., Angele, P., Järvinen, T. A. H., & Docheva, D. (2018). Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Advanced Drug Delivery Reviews, 129, 352–375. https://doi.org/10.1016/j.addr.2017.12.016.
Walia, B., & Huang, A. H. (2019). Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair. Journal of Orthopaedic Research, 37(6), 1270–1280. https://doi.org/10.1002/jor.24156.
Komatsu, I., Wang, J. H. C., Iwasaki, K., Shimizu, T., & Okano, T. (2016). The effect of tendon stem/progenitor cell (TSC) sheet on the early tendon healing in a rat Achilles tendon injury model. Acta Biomaterialia, 42, 136–146. https://doi.org/10.1016/j.actbio.2016.06.026.
Yan, Z., Yin, H., Brochhausen, C., Pfeifer, C. G., Alt, V., & Docheva, D. (2020). Aged tendon stem/progenitor cells are less competent to form 3D tendon Organoids due to cell autonomous and matrix production deficits. Frontiers in Bioengineering and Biotechnology, 8, 406. https://doi.org/10.3389/fbioe.2020.00406.
Liu, Y., Feng, L., Xu, J., Yang, Z., Wu, T., Zhang, J., Shi, L., Zhu, D., Zhang, J., & Li, G. (2019). MiR-378a suppresses tenogenic differentiation and tendon repair by targeting at TGF-beta2. Stem Cell Research & Therapy, 10(1), 108. https://doi.org/10.1186/s13287-019-1216-y.
Docheva, D., Müller, S. A., Majewski, M., & Evans, C. H. (2015). Biologics for tendon repair. Advanced Drug Delivery Reviews, 84, 222–239. https://doi.org/10.1016/j.addr.2014.11.015.
Yin, H., Strunz, F., Yan, Z., Lu, J., Brochhausen, C., Kiderlen, S., Clausen-Schaumann, H., Wang, X., Gomes, M. E., Alt, V., & Docheva, D. (2020). Three-dimensional self-assembling nanofiber matrix rejuvenates aged/degenerative human tendon stem/progenitor cells. Biomaterials, 236, 119802. https://doi.org/10.1016/j.biomaterials.2020.119802.
Yan, Z., Yin, H., Nerlich, M., Pfeifer, C. G., & Docheva, D. (2018). Boosting tendon repair: Interplay of cells, growth factors and scaffold-free and gel-based carriers. J Exp Orthop, 5(1), 1. https://doi.org/10.1186/s40634-017-0117-1.
Tempfer, H., & Traweger, A. (2015). Tendon vasculature in health and disease. Frontiers in Physiology, 6, 330. https://doi.org/10.3389/fphys.2015.00330.
Wang, Y., He, G., Tang, H., Shi, Y., Zhu, M., Kang, X., Bian, X., Lyu, J., Zhou, M., Yang, M., Mu, M., Chen, W., Zhou, B., Yuan, C., Zhang, J., & Tang, K. (2020). Aspirin promotes tenogenic differentiation of tendon stem cells and facilitates tendinopathy healing through regulating the GDF7/Smad1/5 signaling pathway. Journal of Cellular Physiology, 235(5), 4778–4789. https://doi.org/10.1002/jcp.29355.
Chen, E., Yang, L., Ye, C., Zhang, W., Ran, J., Xue, D., Wang, Z., Pan, Z., & Hu, Q. (2018). An asymmetric chitosan scaffold for tendon tissue engineering: In vitro and in vivo evaluation with rat tendon stem/progenitor cells. Acta Biomaterialia, 73, 377–387. https://doi.org/10.1016/j.actbio.2018.04.027.
Kiderlen, S., Polzer, C., Rädler, J. O., Docheva, D., Clausen-Schaumann, H., & Sudhop, S. (2019). Age related changes in cell stiffness of tendon stem/progenitor cells and a rejuvenating effect of ROCK-inhibition. Biochemical and Biophysical Research Communications, 509(3), 839–844. https://doi.org/10.1016/j.bbrc.2019.01.027.
Tarafder, S., Ricupero, C., Minhas, S., Yu, R. J., Alex, A. D., & Lee, C. H. (2019). A combination of Oxo-M and 4-PPBP as a potential regenerative therapeutics for tendon injury. Theranostics, 9(14), 4241–4254. https://doi.org/10.7150/thno.35285.
Shen, H., Jayaram, R., Yoneda, S., Linderman, S. W., Sakiyama-Elbert, S. E., Xia, Y., Gelberman, R. H., & Thomopoulos, S. (2018). The effect of adipose-derived stem cell sheets and CTGF on early flexor tendon healing in a canine model. Scientific Reports, 8(1), 11078. https://doi.org/10.1038/s41598-018-29474-8.
Bi, Y., Ehirchiou, D., Kilts, T. M., Inkson, C. A., Embree, M. C., Sonoyama, W., Li, L., Leet, A. I., Seo, B. M., Zhang, L., Shi, S., & Young, M. F. (2007). Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Medicine, 13(10), 1219–1227. https://doi.org/10.1038/nm1630.
Wu, Y. F., Chen, C., Tang, J. B., & Mao, W. F. (2020). Growth and stem cell characteristics of tendon-derived cells with different initial seeding densities: An in vitro study in mouse flexor tendon cells. Stem Cells and Development, 29(15), 1016–1025. https://doi.org/10.1089/scd.2020.0036.
Youngstrom, D. W., LaDow, J. E., & Barrett, J. G. (2016). Tenogenesis of bone marrow-, adipose-, and tendon-derived stem cells in a dynamic bioreactor. Connective Tissue Research, 57(6), 454–465. https://doi.org/10.3109/03008207.2015.1117458.
Guo, J., Chan, K. M., Zhang, J. F., & Li, G. (2016). Tendon-derived stem cells undergo spontaneous tenogenic differentiation. Experimental Cell Research, 341(1), 1–7. https://doi.org/10.1016/j.yexcr.2016.01.007.
Li, K., Deng, Y., Deng, G., Chen, P., Wang, Y., Wu, H., Ji, Z., Yao, Z., Zhang, X., Yu, B., & Zhang, K. (2020). High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Research & Therapy, 11(1), 131. https://doi.org/10.1186/s13287-020-01643-5.
Shi, L., et al. (2019). Impaired function of tendon-derived stem cells in experimental diabetes mellitus rat tendons: Implications for cellular mechanism of diabetic tendon disorder. Stem Cell Research & Therapy, 10(1), 27. https://doi.org/10.1186/s13287-018-1108-6.
Han, P., Cui, Q., Lu, W., Yang, S., Shi, M., Li, Z., Gao, P., Xu, B., & Li, Z. (2019). Hepatocyte growth factor plays a dual role in tendon-derived stem cell proliferation, migration, and differentiation. Journal of Cellular Physiology, 234(10), 17382–17391. https://doi.org/10.1002/jcp.28360.
Liu, Q., Zhu, Y., Amadio, P. C., Moran, S. L., Gingery, A., & Zhao, C. (2018). Isolation and characterization of multipotent Turkey tendon-derived stem cells. Stem Cells International, 2018, 3697971–3697910. https://doi.org/10.1155/2018/3697971.
Yang, J., Zhao, Q., Wang, K., Ma, C., Liu, H., Liu, Y., & Guan, W. (2018). Isolation, culture and biological characteristics of multipotent porcine tendon-derived stem cells. International Journal of Molecular Medicine, 41(6), 3611–3619. https://doi.org/10.3892/ijmm.2018.3545.
Chen, P., Chen, Z., Mitchell, C., Gao, J., Chen, L., Wang, A., Leys, T., Landao-Bassonga, E., Zheng, Q., Wang, T., & Zheng, M. (2021). Intramuscular injection of Botox causes tendon atrophy by induction of senescence of tendon-derived stem cells. Stem Cell Research & Therapy, 12(1), 38. https://doi.org/10.1186/s13287-020-02084-w.
Rui, Y. F., Lui, P. P. Y., Wong, Y. M., Tan, Q., & Chan, K. M. (2013). Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells and Development, 22(7), 1076–1085. https://doi.org/10.1089/scd.2012.0555.
Zhang, C., Zhu, J., Zhou, Y., Thampatty, B. P., & Wang, J. H. C. (2019). Tendon stem/progenitor cells and their interactions with extracellular matrix and mechanical loading. Stem Cells International, 2019, 3674647–3674610. https://doi.org/10.1155/2019/3674647.
Thampatty, B. P., & Wang, J. H. (2018). Mechanobiology of young and aging tendons: In vivo studies with treadmill running. Journal of Orthopaedic Research, 36(2), 557–565. https://doi.org/10.1002/jor.23761.
Zheng, Z., Ran, J., Chen, W., Hu, Y., Zhu, T., Chen, X., Yin, Z., Heng, B. C., Feng, G., le, H., Tang, C., Huang, J., Chen, Y., Zhou, Y., Dominique, P., Shen, W., & Ouyang, H. W. (2017). Alignment of collagen fiber in knitted silk scaffold for functional massive rotator cuff repair. Acta Biomaterialia, 51, 317–329. https://doi.org/10.1016/j.actbio.2017.01.041.
Wu, H., Zhao, G., Zu, H., Wang, J. H. C., & Wang, Q. M. (2015). Aging-related viscoelasticity variation of tendon stem cells (TSCs) characterized by quartz thickness shear mode (TSM) resonators. Sens Actuators (Warrendale Pa), 210, 369–380. https://doi.org/10.1016/j.snb.2014.12.117.
Lui, P. P. Y., & Wong, C. M. (2019). Biology of tendon stem cells and tendon in aging. Frontiers in Genetics, 10, 1338. https://doi.org/10.3389/fgene.2019.01338.
Rui, Y. F., Chen, M. H., Li, Y. J., Xiao, L. F., Geng, P., Wang, P., Xu, Z. Y., Zhang, X. P., & Dai, G. C. (2019). CTGF attenuates tendon-derived stem/progenitor cell aging. Stem Cells International, 2019, 6257537–6257512. https://doi.org/10.1155/2019/6257537.
Zhang, J., & Wang, J. H. (2015). Moderate exercise mitigates the detrimental effects of aging on tendon stem cells. PLoS One, 10(6), e0130454. https://doi.org/10.1371/journal.pone.0130454.
Popov, C., Kohler, J., & Docheva, D. (2015). Activation of EphA4 and EphB2 reverse signaling restores the age-associated reduction of self-renewal, migration, and actin turnover in human tendon stem/progenitor cells. Frontiers in Aging Neuroscience, 7, 246. https://doi.org/10.3389/fnagi.2015.00246.
Han, W., Wang, B., Liu, J., & Chen, L. (2017). The p16/miR-217/EGR1 pathway modulates age-related tenogenic differentiation in tendon stem/progenitor cells. Acta Biochim Biophys Sin (Shanghai), 49(11), 1015–1021. https://doi.org/10.1093/abbs/gmx104.
Chen, L., Liu, J., Tao, X., Wang, G., Wang, Q., & Liu, X. (2015). The role of Pin1 protein in aging of human tendon stem/progenitor cells. Biochemical and Biophysical Research Communications, 464(2), 487–492. https://doi.org/10.1016/j.bbrc.2015.06.163.
Hu, C., Zhang, Y., Tang, K., Luo, Y., Liu, Y., & Chen, W. (2017). Downregulation of CITED2 contributes to TGFbeta-mediated senescence of tendon-derived stem cells. Cell and Tissue Research, 368(1), 93–104. https://doi.org/10.1007/s00441-016-2552-1.
Chen, M., Li, Y., Xiao, L., Dai, G., Lu, P., Wang, Y., & Rui, Y. (2020). AQP1 modulates tendon stem/progenitor cells senescence during tendon aging. Cell Death & Disease, 11(3), 193. https://doi.org/10.1038/s41419-020-2386-3.
Xu, H., & Liu, F. (2018). Downregulation of FOXP1 correlates with tendon stem/progenitor cells aging. Biochemical and Biophysical Research Communications, 504(1), 96–102. https://doi.org/10.1016/j.bbrc.2018.08.136.
Kim, S. J., Tatman, P. D., Song, D. H., Gee, A. O., Kim, D. H., & Kim, S. J. (2018). Nanotopographic cues and stiffness control of tendon-derived stem cells from diverse conditions. International Journal of Nanomedicine, 13, 7217–7227. https://doi.org/10.2147/IJN.S181743.
Lu, C. C., Zhang, T., Reisdorf, R. L., Amadio, P. C., An, K. N., Moran, S. L., Gingery, A., & Zhao, C. (2019). Biological analysis of flexor tendon repair-failure stump tissue: A potential recycling of tissue for tendon regeneration. Bone Joint Res, 8(6), 232–245. https://doi.org/10.1302/2046-3758.86.BJR-2018-0239.R1.
Yang, D., Wei, Y., Lu, Q., Qin, D., Zhang, M., du, X., Xu, W., Yu, X., He, C., Li, N., Peng, S., Li, G., & Hua, J. (2020). Melatonin alleviates LPS-induced endoplasmic reticulum stress and inflammation in spermatogonial stem cells. Journal of Cellular Physiology. https://doi.org/10.1002/jcp.30088.
Ha, T. W., Jeong, J. H., Shin, H. S., Kim, H. K., Im, J. S., Song, B. H., Hanna, J., Oh, J. S., Woo, D. H., Han, J., & Lee, M. R. (2020). Characterization of endoplasmic reticulum (ER) in human pluripotent stem cells revealed increased susceptibility to cell death upon ER stress. Cells, 9(5). https://doi.org/10.3390/cells9051078.
Tan, Q., Lui, P. P. Y., Rui, Y. F., & Wong, Y. M. (2012). Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Engineering. Part A, 18(7–8), 840–851. https://doi.org/10.1089/ten.TEA.2011.0362.
Qi, F., et al. (2020). From the perspective of embryonic tendon development: Various cells applied to tendon tissue engineering. Ann Transl Med, 8(4), 131. https://doi.org/10.21037/atm.2019.12.78.
Zheng, Y. L. (2016). Some ethical concerns about human induced pluripotent stem cells. Science and Engineering Ethics, 22(5), 1277–1284. https://doi.org/10.1007/s11948-015-9693-6.
Yamanaka, S. (2020). Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell, 27(4), 523–531. https://doi.org/10.1016/j.stem.2020.09.014.
Guo, R., Gao, L., & Xu, B. (2018). Current evidence of adult stem cells to enhance anterior cruciate ligament treatment: A systematic review of animal trials. Arthroscopy, 34(1), 331–340 e332. https://doi.org/10.1016/j.arthro.2017.07.010.
Burk, J., Ribitsch, I., Gittel, C., Juelke, H., Kasper, C., Staszyk, C., & Brehm, W. (2013). Growth and differentiation characteristics of equine mesenchymal stromal cells derived from different sources. Veterinary Journal, 195(1), 98–106. https://doi.org/10.1016/j.tvjl.2012.06.004.
Liu, C., Luo, J. W., Zhang, K. K., Lin, L. X., Liang, T., Luo, Z. P., Zhuang, Y. Q., & Sun, Y. L. (2018). Tendon-derived stem cell differentiation in the degenerative tendon microenvironment. Stem Cells International, 2018, 2613821–2613812. https://doi.org/10.1155/2018/2613821.
Kwan, C. K., Fu, S. C., & Yung, P. S. H. (2020). A high glucose level stimulate inflammation and weaken pro-resolving response in tendon cells - a possible factor contributing to tendinopathy in diabetic patients. Asia Pac J Sports Med Arthrosc Rehabil Technol, 19, 1–6. https://doi.org/10.1016/j.asmart.2019.10.002.
Yang, J., Zhao, Q., Wang, K., Liu, H., Ma, C., Huang, H., & Liu, Y. (2016). Isolation and biological characterization of tendon-derived stem cells from fetal bovine. Vitro Cell Dev Biol Anim, 52(8), 846–856. https://doi.org/10.1007/s11626-016-0043-z.
Williamson, K. A., Lee, K. J., Humphreys, W. J. E., Comerford, E. J. V., Clegg, P. D., & Canty-Laird, E. G. (2015). Restricted differentiation potential of progenitor cell populations obtained from the equine superficial digital flexor tendon (SDFT). Journal of Orthopaedic Research, 33(6), 849–858. https://doi.org/10.1002/jor.22891.
Mienaltowski, M. J., Adams, S. M., & Birk, D. E. (2014). Tendon proper- and peritenon-derived progenitor cells have unique tenogenic properties. Stem Cell Research & Therapy, 5(4), 86. https://doi.org/10.1186/scrt475.
Lee, K. J., Clegg, P. D., Comerford, E. J., & Canty-Laird, E. G. (2018). A comparison of the stem cell characteristics of murine tenocytes and tendon-derived stem cells. BMC Musculoskeletal Disorders, 19(1), 116. https://doi.org/10.1186/s12891-018-2038-2.
Chen, J., Zhang, W., Liu, Z., Zhu, T., Shen, W., Ran, J., Tang, Q., Gong, X., Backman, L. J., Chen, X., Chen, X., Wen, F., & Ouyang, H. (2016). Characterization and comparison of post-natal rat Achilles tendon-derived stem cells at different development stages. Scientific Reports, 6, 22946. https://doi.org/10.1038/srep22946.
Lee, W. Y., et al. (2012). Hypoxia-mediated efficient expansion of human tendon-derived stem cells in vitro. Tissue Engineering. Part A, 18(5–6), 484–498. https://doi.org/10.1089/ten.TEA.2011.0130.
Zhang, J., & Wang, J. H. (2013). Human tendon stem cells better maintain their stemness in hypoxic culture conditions. PLoS One, 8(4), e61424. https://doi.org/10.1371/journal.pone.0061424.
Yu, Y., et al. (2017). Effect of hypoxia on self-renewal capacity and differentiation in human tendon-derived stem cells. Medical Science Monitor, 23, 1334–1339. https://doi.org/10.12659/msm.903892.
Li, P., Xu, Y., Gan, Y., Song, L., Zhang, C., Wang, L., & Zhou, Q. (2016). Role of the ERK1/2 signaling pathway in Osteogenesis of rat tendon-derived stem cells in normoxic and hypoxic cultures. International Journal of Medical Sciences, 13(8), 629–637. https://doi.org/10.7150/ijms.16045.
Zhang, J., et al. (2016). Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats. Oncotarget, 7(8), 8498–8512. https://doi.org/10.18632/oncotarget.7381.
Qiu, S., Jia, Y., Tang, J., Liu, X., Hu, H., Wu, T., & Chai, Y. (2018). Von Hippel-Lindau (VHL) protein antagonist, VH298, promotes functional activities of tendon-derived stem cells and accelerates healing of entheses in rats by inhibiting ubiquitination of hydroxy-HIF-1alpha. Biochemical and Biophysical Research Communications, 505(4), 1063–1069. https://doi.org/10.1016/j.bbrc.2018.09.172.
Chen, S., et al. (2018). Interleukin-6 promotes proliferation but inhibits Tenogenic differentiation via the Janus kinase/signal transducers and activators of transcription 3 (JAK/STAT3) pathway in tendon-derived stem cells. Medical Science Monitor, 24, 1567–1573. https://doi.org/10.12659/msm.908802.
Wu, T., Liu, S., Wen, G., Xu, J., Yu, Y., & Chai, Y. (2017). Celastrol improves self-renewal and differentiation of human tendon-derived stem cells by suppressing Smad7 through hypoxia. Stem Cell Research & Therapy, 8(1), 274. https://doi.org/10.1186/s13287-017-0724-x.
Lee, Y. W., Fu, S. C., Yeung, M. Y., Lau, C. M. L., Chan, K. M., & Hung, L. K. (2017). Effects of redox modulation on cell proliferation, viability, and migration in cultured rat and human tendon progenitor cells. Oxidative Medicine and Cellular Longevity, 2017, 8785042–8785048. https://doi.org/10.1155/2017/8785042.
Han, W., Chen, L., Liu, J., & Guo, A. (2017). Enhanced tenogenic differentiation and tendon-like tissue formation by CHIP overexpression in tendon-derived stem cells. Acta Biochim Biophys Sin (Shanghai), 49(4), 311–317. https://doi.org/10.1093/abbs/gmx005.
Han, P., Cui, Q., Yang, S., Wang, H., Gao, P., & Li, Z. (2017). Tumor necrosis factor-alpha and transforming growth factor-beta1 facilitate differentiation and proliferation of tendon-derived stem cells in vitro. Biotechnology Letters, 39(5), 711–719. https://doi.org/10.1007/s10529-017-2296-3.
Tarafder, S., Chen, E., Jun, Y., Kao, K., Sim, K. H., Back, J., Lee, F. Y., & Lee, C. H. (2017). Tendon stem/progenitor cells regulate inflammation in tendon healing via JNK and STAT3 signaling. The FASEB Journal, 31(9), 3991–3998. https://doi.org/10.1096/fj.201700071R.
Lui, P. P. (2015). Markers for the identification of tendon-derived stem cells in vitro and tendon stem cells in situ - update and future development. Stem Cell Research & Therapy, 6(1), 106. https://doi.org/10.1186/s13287-015-0097-y.
Zhang, X., Lin, Y. C., Rui, Y. F., Xu, H. L., Chen, H., Wang, C., & Teng, G. J. (2016). Therapeutic roles of tendon stem/progenitor cells in Tendinopathy. Stem Cells International, 2016, 4076578–4076514. https://doi.org/10.1155/2016/4076578.
Liu, C., Luo, J. W., Liang, T., Lin, L. X., Luo, Z. P., Zhuang, Y. Q., & Sun, Y. L. (2018). Matrix stiffness regulates the differentiation of tendon-derived stem cells through FAK-ERK1/2 activation. Experimental Cell Research, 373(1–2), 62–70. https://doi.org/10.1016/j.yexcr.2018.08.023.
Liu, Y., Xu, J., Xu, L., Wu, T., Sun, Y., Lee, Y. W., Wang, B., Chan, H. C., Jiang, X., Zhang, J., & Li, G. (2017). Cystic fibrosis transmembrane conductance regulator mediates tenogenic differentiation of tendon-derived stem cells and tendon repair: Accelerating tendon injury healing by intervening in its downstream signaling. The FASEB Journal, 31(9), 3800–3815. https://doi.org/10.1096/fj.201601181R.
Lejard, V., Blais, F., Guerquin, M. J., Bonnet, A., Bonnin, M. A., Havis, E., Malbouyres, M., Bidaud, C. B., Maro, G., Gilardi-Hebenstreit, P., Rossert, J., Ruggiero, F., & Duprez, D. (2011). EGR1 and EGR2 involvement in vertebrate tendon differentiation. The Journal of Biological Chemistry, 286(7), 5855–5867. https://doi.org/10.1074/jbc.M110.153106.
Jiang, H., Chen, Y., Chen, G., Tian, X., Tang, J., Luo, L., Huang, M., Yan, B., Ao, X., Zhou, W., Wang, L., Bai, X., Zhang, Z., Wang, L., & Xian, C. J. (2018). Leptin accelerates the pathogenesis of heterotopic ossification in rat tendon tissues via mTORC1 signaling. Journal of Cellular Physiology, 233(2), 1017–1028. https://doi.org/10.1002/jcp.25955.
Qin, S., Wang, W., Liu, Z., Hua, X., Fu, S. C., Dong, F., Li, A., Liu, Z., Wang, P., Dai, L., Liang, P., Zhang, J., Cao, W., Xiong, X., Chen, H., & Xu, J. (2020). Fibrochondrogenic differentiation potential of tendon-derived stem/progenitor cells from human patellar tendon. J Orthop Translat, 22, 101–108. https://doi.org/10.1016/j.jot.2019.08.006.
Cheng, X., Xu, J., Hu, Z., Jiang, J., Wang, Z., & Lu, M. (2020). Dual-modal magnetic resonance and photoacoustic tracking and outcome of transplanted tendon stem cells in the rat rotator cuff injury model. Scientific Reports, 10(1), 13954. https://doi.org/10.1038/s41598-020-69214-5.
Lu, M., Cheng, X., Jiang, J., Li, T. T., Zhang, Z., Tsauo, C., Liu, Y., & Wang, Z. (2018). Dual-modal photoacoustic and magnetic resonance tracking of tendon stem cells with PLGA/iron oxide microparticles in vitro. PLoS One, 13(4), e0193362. https://doi.org/10.1371/journal.pone.0193362.
Rajpar, I., & Barrett, J. G. (2020). Multi-differentiation potential is necessary for optimal tenogenesis of tendon stem cells. Stem Cell Research & Therapy, 11(1), 152. https://doi.org/10.1186/s13287-020-01640-8.
Brown, J. P., Galassi, T. V., Stoppato, M., Schiele, N. R., & Kuo, C. K. (2015). Comparative analysis of mesenchymal stem cell and embryonic tendon progenitor cell response to embryonic tendon biochemical and mechanical factors. Stem Cell Research & Therapy, 6, 89. https://doi.org/10.1186/s13287-015-0043-z.
Liu, J., Tao, X., Chen, L., Han, W., Zhou, Y., & Tang, K. (2015). CTGF positively regulates BMP12 induced tenogenic differentiation of tendon stem cells and signaling. Cellular Physiology and Biochemistry, 35(5), 1831–1845. https://doi.org/10.1159/000373994.
Xu, K., Sun, Y., Kh al-ani, M., Wang, C., Sha, Y., Sung, K. L. P., Dong, N., Qiu, X., & Yang, L. (2018). Synergistic promoting effects of bone morphogenetic protein 12/connective tissue growth factor on functional differentiation of tendon derived stem cells and patellar tendon window defect regeneration. Journal of Biomechanics, 66, 95–102. https://doi.org/10.1016/j.jbiomech.2017.11.004.
Seabaugh, K. A., Thoresen, M., & Giguère, S. (2017). Extracorporeal shockwave therapy increases growth factor release from equine platelet-rich plasma in vitro. Front Vet Sci, 4, 205. https://doi.org/10.3389/fvets.2017.00205.
Xu, K., al-ani, M. K., Sun, Y., Xu, W., Pan, L., Song, Y., Xu, Z. L., Pan, X., & Yang, L. (2017). Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. Journal of Tissue Engineering and Regenerative Medicine, 11(4), 1173–1184. https://doi.org/10.1002/term.2020.
Zhang, L., Chen, S., Chang, P., Bao, N., Yang, C., Ti, Y., Zhou, L., & Zhao, J. (2016). Harmful effects of leukocyte-rich platelet-rich plasma on rabbit tendon stem cells in vitro. The American Journal of Sports Medicine, 44(8), 1941–1951. https://doi.org/10.1177/0363546516644718.
Zhou, Y., Zhang, J., Wu, H., Hogan, M. C. V., & Wang, J. H. C. (2015). The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Research & Therapy, 6(1), 173. https://doi.org/10.1186/s13287-015-0172-4.
Li, X., Pongkitwitoon, S., Lu, H., Lee, C., Gelberman, R., & Thomopoulos, S. (2019). CTGF induces tenogenic differentiation and proliferation of adipose-derived stromal cells. Journal of Orthopaedic Research, 37(3), 574–582. https://doi.org/10.1002/jor.24248.
Lee, C. H., Lee, F. Y., Tarafder, S., Kao, K., Jun, Y., Yang, G., & Mao, J. J. (2015). Harnessing endogenous stem/progenitor cells for tendon regeneration. The Journal of Clinical Investigation, 125(7), 2690–2701. https://doi.org/10.1172/JCI81589.
Vigano, M., et al. (2017). Different culture conditions affect the growth of human tendon stem/progenitor cells (TSPCs) within a mixed tendon cells (TCs) population. J Exp Orthop, 4(1), 8. https://doi.org/10.1186/s40634-017-0082-8.
Deng, G., Li, K., Chen, S., Chen, P., Zheng, H., Yu, B., & Zhang, K. (2018). Interleukin10 promotes proliferation and migration, and inhibits tendon differentiation via the JAK/Stat3 pathway in tendonderived stem cells in vitro. Molecular Medicine Reports, 18(6), 5044–5052. https://doi.org/10.3892/mmr.2018.9547.
Zhang, K., Asai, S., Yu, B., & Enomoto-Iwamoto, M. (2015). IL-1beta irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochemical and Biophysical Research Communications, 463(4), 667–672. https://doi.org/10.1016/j.bbrc.2015.05.122.
Zhang, Y. J., Qing, Q., Zhang, Y. J., Ning, L. J., Cui, J., Yao, X., Luo, J. C., Ding, W., & Qin, T. W. (2019). Enhancement of tenogenic differentiation of rat tendon-derived stem cells by biglycan. Journal of Cellular Physiology, 234, 15898–15910. https://doi.org/10.1002/jcp.28247.
Yu, Y., Chen, Y., Zheng, Y. J., Weng, Q. H., Zhu, S. P., & Zhou, D. S. (2020). LncRNA TUG1 promoted osteogenic differentiation through promoting bFGF ubiquitination. Vitro Cell Dev Biol Anim, 56(1), 42–48. https://doi.org/10.1007/s11626-019-00410-y.
Geng, Y., Zhao, X., Xu, J., Zhang, X., Hu, G., Fu, S. C., Dai, K., Chen, X., Patrick, Y. H., & Zhang, X. (2020). Overexpression of mechanical sensitive miR-337-3p alleviates ectopic ossification in rat tendinopathy model via targeting IRS1 and Nox4 of tendon-derived stem cells. Journal of Molecular Cell Biology, 12(4), 305–317. https://doi.org/10.1093/jmcb/mjz030.
Lu, Y. F., Liu, Y., Fu, W. M., Xu, J., Wang, B., Sun, Y. X., Wu, T. Y., Xu, L. L., Chan, K. M., Zhang, J. F., & Li, G. (2017). Long noncoding RNA H19 accelerates tenogenic differentiation and promotes tendon healing through targeting miR-29b-3p and activating TGF-beta1 signaling. The FASEB Journal, 31(3), 954–964. https://doi.org/10.1096/fj.201600722R.
Yu, Y., Chen, Y., Zhang, X., Lu, X., Hong, J., Guo, X., & Zhou, D. (2018). Knockdown of lncRNA KCNQ1OT1 suppresses the adipogenic and osteogenic differentiation of tendon stem cell via downregulating miR-138 target genes PPARgamma and RUNX2. Cell Cycle, 17(19–20), 2374–2385. https://doi.org/10.1080/15384101.2018.1534510.
Chen, Y., et al. (2020). Targeted pathological collagen delivery of sustained-release rapamycin to prevent heterotopic ossification. Science Advances, 6(18), eaay9526. https://doi.org/10.1126/sciadv.aay9526.
Bergante, S., Creo, P., Piccoli, M., Ghiroldi, A., Menon, A., Cirillo, F., Rota, P., Monasky, M. M., Ciconte, G., Pappone, C., Randelli, P., & Anastasia, L. (2018). GM1 Ganglioside promotes Osteogenic differentiation of human tendon stem cells. Stem Cells International, 2018, 4706943–4706948. https://doi.org/10.1155/2018/4706943.
Tao, X., Liu, J., Chen, L., Zhou, Y., & Tang, K. (2015). EGR1 induces tenogenic differentiation of tendon stem cells and promotes rabbit rotator cuff repair. Cellular Physiology and Biochemistry, 35(2), 699–709. https://doi.org/10.1159/000369730.
Wang, B., Guo, J., Feng, L., Suen, C. W., Fu, W. M., Zhang, J. F., & Li, G. (2016). MiR124 suppresses collagen formation of human tendon derived stem cells through targeting egr1. Experimental Cell Research, 347(2), 360–366. https://doi.org/10.1016/j.yexcr.2016.08.018.
Liu, J., Han, W., Chen, L., & Tang, K. (2016). Mechanism of osteogenic and adipogenic differentiation of tendon stem cells induced by sirtuin 1. Molecular Medicine Reports, 14(2), 1643–1648. https://doi.org/10.3892/mmr.2016.5417.
Xu, L., Xu, K., Wu, Z., Chen, Z., He, Y., Ma, C., Moqbel, S. A. A., Ran, J., Zhang, C., Wu, L., & Xiong, Y. (2020). Pioglitazone attenuates advanced glycation end products-induced apoptosis and calcification by modulating autophagy in tendon-derived stem cells. Journal of Cellular and Molecular Medicine, 24(3), 2240–2251. https://doi.org/10.1111/jcmm.14901.
Li, K., Deng, G., Deng, Y., Chen, S., Wu, H., Cheng, C., Zhang, X., Yu, B., & Zhang, K. (2019). High cholesterol inhibits tendon-related gene expressions in tendon-derived stem cells through reactive oxygen species-activated nuclear factor-kappaB signaling. Journal of Cellular Physiology, 234(10), 18017–18028. https://doi.org/10.1002/jcp.28433.
Lin, Y. C., et al. (2017). The effects of high glucose on tendon-derived stem cells: Implications of the pathogenesis of diabetic tendon disorders. Oncotarget, 8(11), 17518–17528. https://doi.org/10.18632/oncotarget.15418.
Ning, L. J., Zhang, Y. J., Zhang, Y., Qing, Q., Jiang, Y. L., Yang, J. L., Luo, J. C., & Qin, T. W. (2015). The utilization of decellularized tendon slices to provide an inductive microenvironment for the proliferation and tenogenic differentiation of stem cells. Biomaterials, 52, 539–550. https://doi.org/10.1016/j.biomaterials.2015.02.061.
Zhang, J., Li, B., & Wang, J. H. C. (2011). The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials, 32(29), 6972–6981. https://doi.org/10.1016/j.biomaterials.2011.05.088.
Yin, Z., Chen, X., Zhu, T., Hu, J. J., Song, H. X., Shen, W. L., Jiang, L. Y., Heng, B. C., Ji, J. F., & Ouyang, H. W. (2013). The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomaterialia, 9(12), 9317–9329. https://doi.org/10.1016/j.actbio.2013.07.022.
Jiang, D., et al. (2018). Effects of young extracellular matrix on the biological characteristics of aged tendon stem cells. Advances in Clinical and Experimental Medicine, 27(12), 1625–1630. https://doi.org/10.17219/acem/75503.
Popov, C., Burggraf, M., Kreja, L., Ignatius, A., Schieker, M., & Docheva, D. (2015). Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Molecular Biology, 16, 6. https://doi.org/10.1186/s12867-015-0036-6.
Wang, T., Thien, C., Wang, C., Ni, M., Gao, J., Wang, A., Jiang, Q., Tuan, R. S., Zheng, Q., & Zheng, M. H. (2018). 3D uniaxial mechanical stimulation induces tenogenic differentiation of tendon-derived stem cells through a PI3K/AKT signaling pathway. The FASEB Journal, 32(9), 4804–4814. https://doi.org/10.1096/fj.201701384R.
Chen, Z., Chen, P., Ruan, R., Chen, L., Yuan, J., Wood, D., Wang, T., & Zheng, M. H. (2020). Applying a three-dimensional uniaxial mechanical stimulation bioreactor system to induce Tenogenic differentiation of tendon-derived stem cells. Journal of Visualized Experiments, 162. https://doi.org/10.3791/61278.
Xu, Y., Wang, Q., Li, Y., Gan, Y., Li, P., Li, S., Zhou, Y., & Zhou, Q. (2015). Cyclic tensile strain induces Tenogenic differentiation of tendon-derived stem cells in bioreactor culture. BioMed Research International, 2015, 790804–790813. https://doi.org/10.1155/2015/790804.
Chen, H., Ge, H. A., Wu, G. B., Cheng, B., Lu, Y., & Jiang, C. (2016). Autophagy prevents oxidative stress-induced loss of self-renewal capacity and Stemness in human tendon stem cells by reducing ROS accumulation. Cellular Physiology and Biochemistry, 39(6), 2227–2238. https://doi.org/10.1159/000447916.
Hu, J. J., Yin, Z., Shen, W. L., Xie, Y. B., Zhu, T., Lu, P., Cai, Y. Z., Kong, M. J., Heng, B. C., Zhou, Y. T., Chen, W. S., Chen, X., & Ouyang, H. W. (2016). Pharmacological regulation of in situ tissue stem cells differentiation for soft tissue calcification treatment. Stem Cells, 34(4), 1083–1096. https://doi.org/10.1002/stem.2306.
Schmalzl, J., Plumhoff, P., Gilbert, F., Gohlke, F., Konrads, C., Brunner, U., Jakob, F., Ebert, R., & Steinert, A. F. (2019). Tendon-derived stem cells from the long head of the biceps tendon: Inflammation does not affect the regenerative potential. Bone Joint Res, 8(9), 414–424. https://doi.org/10.1302/2046-3758.89.BJR-2018-0214.R2.
Chen, W., Tang, H., Zhou, M., Hu, C., Zhang, J., & Tang, K. (2015). Dexamethasone inhibits the differentiation of rat tendon stem cells into tenocytes by targeting the scleraxis gene. The Journal of Steroid Biochemistry and Molecular Biology, 152, 16–24. https://doi.org/10.1016/j.jsbmb.2015.04.010.
Zhang, J., Keenan, C., & Wang, J. H. C. (2013). The effects of dexamethasone on human patellar tendon stem cells: Implications for dexamethasone treatment of tendon injury. Journal of Orthopaedic Research, 31(1), 105–110. https://doi.org/10.1002/jor.22193.
Wang, Y., He, G., Tang, H., Shi, Y., Kang, X., Lyu, J., Zhu, M., Zhou, M., Yang, M., Mu, M., Chen, W., Zhou, B., Zhang, J., & Tang, K. (2019). Aspirin inhibits inflammation and scar formation in the injury tendon healing through regulating JNK/STAT-3 signalling pathway. Cell Proliferation, 52(4), e12650. https://doi.org/10.1111/cpr.12650.
Wang, Y., He, G., Wang, F., Zhang, C., Ge, Z., Zheng, X., Deng, H., Yuan, C., Zhou, B., Tao, X., Zhang, J., & Tang, K. (2019). Aspirin inhibits adipogenesis of tendon stem cells and lipids accumulation in rat injury tendon through regulating PTEN/PI3K/AKT signalling. Journal of Cellular and Molecular Medicine, 23(11), 7535–7544. https://doi.org/10.1111/jcmm.14622.
Shi, Z., Wang, Q., & Jiang, D. (2019). Ascorbic acid mitigates the deleterious effects of nicotine on tendon stem cells. Connective Tissue Research, 62, 1–11. https://doi.org/10.1080/03008207.2019.1663349.
Zhou, W., Lin, X., Chu, J., Jiang, T., Zhao, H., Yan, B., & Zhang, Z. (2019). Magnolol prevents ossified tendinopathy by inhibiting PGE2-induced osteogenic differentiation of TDSCs. International Immunopharmacology, 70, 117–124. https://doi.org/10.1016/j.intimp.2019.02.010.
Tian, X., Jiang, H., Chen, Y., Ao, X., Chen, C., Zhang, W., He, F., Liao, X., Jiang, X., Li, T., Zhang, Z., & Zhang, X. (2018). Baicalein accelerates tendon-bone healing via activation of Wnt/beta-catenin signaling pathway in rats. BioMed Research International, 2018, 3849760–3849769. https://doi.org/10.1155/2018/3849760.
Wang, Y., He, G., Guo, Y., Tang, H., Shi, Y., Bian, X., Zhu, M., Kang, X., Zhou, M., Lyu, J., Yang, M., Mu, M., Lai, F., Lu, K., Chen, W., Zhou, B., Zhang, J., & Tang, K. (2019). Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. Journal of Cellular and Molecular Medicine, 23(8), 5475–5485. https://doi.org/10.1111/jcmm.14430.
Yang, Z., et al. (2017). Effect of tendon stem cells in chitosan/beta-Glycerophosphate/collagen hydrogel on Achilles tendon healing in a rat model. Medical Science Monitor, 23, 4633–4643. https://doi.org/10.12659/msm.906747.
Yin, H., Yan, Z., Bauer, R. J., Peng, J., Schieker, M., Nerlich, M., & Docheva, D. (2018). Functionalized thermosensitive hydrogel combined with tendon stem/progenitor cells as injectable cell delivery carrier for tendon tissue engineering. Biomedical Materials, 13(3), 034107. https://doi.org/10.1088/1748-605X/aaadd1.
Chang, D., Shimizu, T., Haraguchi, Y., Gao, S., Sakaguchi, K., Umezu, M., Yamato, M., Liu, Z., & Okano, T. (2015). Time course of cell sheet adhesion to porcine heart tissue after transplantation. PLoS One, 10(10), e0137494. https://doi.org/10.1371/journal.pone.0137494.
Zhang, H., Zhou, Y., Zhang, W., Wang, K., Xu, L., Ma, H., & Deng, Y. (2018). Construction of vascularized tissue-engineered bone with a double-cell sheet complex. Acta Biomaterialia, 77, 212–227. https://doi.org/10.1016/j.actbio.2018.07.024.
Kawanishi, K., Yamato, M., Sakiyama, R., Okano, T., & Nitta, K. (2016). Peritoneal cell sheets composed of mesothelial cells and fibroblasts prevent intra-abdominal adhesion formation in a rat model. Journal of Tissue Engineering and Regenerative Medicine, 10(10), 855–866. https://doi.org/10.1002/term.1860.
Bardag-Gorce, F., Oliva, J., Wood, A., Hoft, R., Pan, D., Thropay, J., Makalinao, A., French, S. W., & Niihara, Y. (2015). Carrier-free cultured autologous oral mucosa epithelial cell sheet (CAOMECS) for corneal epithelium reconstruction: A histological study. The Ocular Surface, 13(2), 150–163. https://doi.org/10.1016/j.jtos.2014.12.003.
Zhang, C., Zhang, E., Yang, L., Tu, W., Lin, J., Yuan, C., Bunpetch, V., Chen, X., & Ouyang, H. (2018). Histone deacetylase inhibitor treated cell sheet from mouse tendon stem/progenitor cells promotes tendon repair. Biomaterials, 172, 66–82. https://doi.org/10.1016/j.biomaterials.2018.03.043.
Liu, Y., Suen, C. W., Zhang, J. F., & Li, G. (2017). Current concepts on tenogenic differentiation and clinical applications. J Orthop Translat, 9, 28–42. https://doi.org/10.1016/j.jot.2017.02.005.
Song, H., Yin, Z., Wu, T., Li, Y., Luo, X., Xu, M., Duan, L., & Li, J. (2018). Enhanced effect of tendon stem/progenitor cells combined with tendon-derived Decellularized extracellular matrix on tendon regeneration. Cell Transplantation, 27(11), 1634–1643. https://doi.org/10.1177/0963689718805383.
Han, F., Zhang, P., Wen, X., Lin, C., & Zhao, P. (2019). Bioactive LbL-assembled multilayer nanofilms upregulate tenogenesis and angiogenesis enabling robust healing of degenerative rotator cuff tendons in vivo. Biomaterials Science, 7(10), 4388–4398. https://doi.org/10.1039/c9bm00413k.
Zhang, C., Wang, X., Zhang, E., Yang, L., Yuan, H., Tu, W., Zhang, H., Yin, Z., Shen, W., Chen, X., Zhang, Y., & Ouyang, H. (2018). An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomaterialia, 66, 141–156. https://doi.org/10.1016/j.actbio.2017.09.036.
Nourissat, G., Berenbaum, F., & Duprez, D. (2015). Tendon injury: From biology to tendon repair. Nature Reviews Rheumatology, 11(4), 223–233. https://doi.org/10.1038/nrrheum.2015.26.
Heinemeier, K. M., Mackey, A. L., Doessing, S., Hansen, M., Bayer, M. L., Nielsen, R. H., Herchenhan, A., Malmgaard-Clausen, N. M., & Kjaer, M. (2012). GH/IGF-I axis and matrix adaptation of the musculotendinous tissue to exercise in humans. Scandinavian Journal of Medicine & Science in Sports, 22(4), e1–e7. https://doi.org/10.1111/j.1600-0838.2012.01459.x.
Liu, X., Chen, W., Zhou, Y., Tang, K., & Zhang, J. (2015). Mechanical tension promotes the Osteogenic differentiation of rat tendon-derived stem cells through the Wnt5a/Wnt5b/JNK signaling pathway. Cellular Physiology and Biochemistry, 36(2), 517–530. https://doi.org/10.1159/000430117.
Wang, Y., Tang, H., He, G., Shi, Y., Kang, X., Lyu, J., Zhou, M., Zhu, M., Zhang, J., & Tang, K. (2018). High concentration of aspirin induces apoptosis in rat tendon stem cells via inhibition of the Wnt/beta-catenin pathway. Cellular Physiology and Biochemistry, 50(6), 2046–2059. https://doi.org/10.1159/000495050.
Chen, W., Tang, H., Liu, X., Zhou, M., Zhang, J., & Tang, K. (2015). Dickkopf1 up-regulation induced by a high concentration of dexamethasone promotes rat tendon stem cells to differentiate into adipocytes. Cellular Physiology and Biochemistry, 37(5), 1738–1749. https://doi.org/10.1159/000438538.
Acknowledgements
The authors thank Mingliang Ji, Yucheng Lin from Department of Orthopaedic Surgery, Zhongda Hospital, School of Medicine, Southeast University.
Code Availability
Not applicable.
Funding
This work was supported by The National Natural Science Foundation of China (Grant numbers 81672159 and 82072427).
Author information
Authors and Affiliations
Contributions
Bing Wei performed the literature search, data analysis, and prepared table, figures and drafted manuscript; Jun Lu had the idea for the article and critically revised the work.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflicts of interest.
Compliance with Ethics Standards
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, B., Lu, J. Characterization of Tendon-Derived Stem Cells and Rescue Tendon Injury. Stem Cell Rev and Rep 17, 1534–1551 (2021). https://doi.org/10.1007/s12015-021-10143-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10143-9