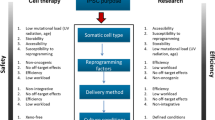
Abstract
Human induced Pluripotent Stem Cells (iPSCs) have enormous potential in understanding developmental biology, disease modeling, drug discovery, and regenerative medicine. The initial human iPSC studies used fibroblasts as a starting cell source to reprogram them; however, it has been identified to be a less appealing somatic cell source by numerous studies due to various reasons. One of the important criteria to achieve efficient reprogramming is determining an appropriate starting somatic cell type to induce pluripotency since the cellular source has a major influence on the reprogramming efficiency, kinetics, and quality of iPSCs. Therefore, numerous groups have explored various somatic cell sources to identify the promising sources for reprogramming into iPSCs with different reprogramming factor combinations. This review provides an overview of promising easily accessible somatic cell sources isolated in non-invasive or minimally invasive manner such as keratinocytes, urine cells, and peripheral blood mononuclear cells used for the generation of human iPSCs derived from healthy and diseased subjects. Notably, iPSCs generated from one of these cell types derived from the patient will offer ethical and clinical advantages. In addition, these promising somatic cell sources have the potential to efficiently generate bona fide iPSCs with improved reprogramming efficiency and faster kinetics. This knowledge will help in establishing strategies for safe and efficient reprogramming and the generation of patient-specific iPSCs from these cell types.
Graphical abstract




Similar content being viewed by others
References
Evans, M. J., & Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature, 292(5819), 154–156. https://doi.org/10.1038/292154a0
Martin, G. R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences, 78(12), 7634–7638. https://doi.org/10.1073/pnas.78.12.7634
Thomson, J. A. (1998). Embryonic Stem Cell Lines Derived from Human Blastocysts. Science, 282(5391), 1145–1147. https://doi.org/10.1126/science.282.5391.1145
Takahashi, K., & Yamanaka, S. (2006). Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell, 126(4), 663–676. https://doi.org/10.1016/j.cell.2006.07.024
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell, 131(5), 861–872. https://doi.org/10.1016/j.cell.2007.11.019
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., & Thomson, J. A. (2007). Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science, 318(5858), 1917–1920. https://doi.org/10.1126/science.1151526
Omole, A. E., & Fakoya, A. O. J. (2018). Ten years of progress and promise of induced pluripotent stem cells: Historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ, 2018 (MAY), 1–47. https://doi.org/10.7717/peerj.4370
Haridhasapavalan, K. K., Borgohain, M. P., Dey, C., Saha, B., Narayan, G., Kumar, S., & Thummer, R. P. (2019). An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene, 686. https://doi.org/10.1016/j.gene.2018.11.069
Borgohain, M. P., Haridhasapavalan, K. K., Dey, C., Adhikari, P., & Thummer, R. P. (2019). An Insight into DNA-free Reprogramming Approaches to Generate Integration-free Induced Pluripotent Stem Cells for Prospective Biomedical Applications. Stem Cell Reviews and Reports, 15(2), 286–313. https://doi.org/10.1007/s12015-018-9861-6
Haridhasapavalan, K. K., Raina, K., Dey, C., Adhikari, P., & Thummer, R. P. (2020). An Insight into Reprogramming Barriers to iPSC Generation. Stem Cell Reviews and Reports, 16(1), 56–81. https://doi.org/10.1007/s12015-019-09931-1
Dey, C., Raina, K., Haridhasapavalan, K. K., Thool, M., Sundaravadivelu, P. K., Adhikari, P., Thummer, R. P. (2021). An overview of reprogramming approaches to derive integration-free induced pluripotent stem cells for prospective biomedical applications. In Recent Advances in iPSC Technology (pp. 231–287). Elsevier. https://doi.org/10.1016/B978-0-12-822231-7.00011-4
Singh, V. K., Kalsan, M., Kumar, N., Saini, A., & Chandra, R. (2015). Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Frontiers in Cell and Developmental Biology, 3. https://doi.org/10.3389/fcell.2015.00002
Menon, S., Shailendra, S., Renda, A., Longaker, M., & Quarto, N. (2016). An Overview of Direct Somatic Reprogramming: The Ins and Outs of iPSCs. International Journal of Molecular Sciences, 17(1), 141. https://doi.org/10.3390/ijms17010141
Liu, G., David, B. T., Trawczynski, M., & Fessler, R. G. (2020). Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Reviews and Reports, 16(1), 3–32. https://doi.org/10.1007/s12015-019-09935-x
Winder, M. L., & Trokovic, R. (2021). Induced pluripotent stem cell derivation from myoblasts. In Cell Sources for iPSCs (pp. 37–55). Elsevier. https://doi.org/10.1016/B978-0-12-822135-8.00009-4
Raab, S., Klingenstein, M., Liebau, S., & Linta, L. (2014). A Comparative View on Human Somatic Cell Sources for iPSC Generation. Stem Cells International, 2014. https://doi.org/10.1155/2014/768391
Saha, B., Krishna Kumar, H., Borgohain, M. P., & Thummer, R. P. (2018). Prospective applications of induced pluripotent stem cells in military medicine. Medical Journal Armed Forces India, 74(4), 313–320. https://doi.org/10.1016/j.mjafi.2018.03.005
Petzendorfer, E., & Guillot, P. V. (2021). Induced pluripotent stem cells derived from amniotic fluid stem cells. In Cell Sources for iPSCs (pp. 1–13). Elsevier. https://doi.org/10.1016/B978-0-12-822135-8.00010-0
Chahine, M. (2021). Lymphoblastoid-derived human-induced pluripotent stem cells. In Cell Sources for iPSCs (pp. 57–70). Elsevier. https://doi.org/10.1016/B978-0-12-822135-8.00005-7
Jamal, M., Bashir, A., Al-Sayegh, M., & Huang, G. T.-J. (2021). Oral tissues as sources for induced pluripotent stem cell derivation and their applications for neural, craniofacial, and dental tissue regeneration. In Cell Sources for iPSCs (pp. 71–106). Elsevier. https://doi.org/10.1016/B978-0-12-822135-8.00007-0
Disler, E. R., Ng, N. W., Nguyen, T. G., Anchan, C. J., Waldman, I. N., & Anchan, R. M. (2021). Induced pluripotent stem cell derived from ovarian tissue. In Cell Sources for iPSCs (pp. 107–135). Elsevier. https://doi.org/10.1016/B978-0-12-822135-8.00011-2
Li, G., Wakao, S., Kuroda, Y., Kushida, Y., & Dezawa, M. (2021). Muse cells as a robust source of induced pluripotent stem cells. In Cell Sources for iPSCs (Vol. 13, pp. 137–161). Elsevier. https://doi.org/10.1016/B978-0-12-822135-8.00006-9
Pellicano, R., Caviglia, G. P., Ribaldone, D. G., Altruda, F., & Fagoonee, S. (2021). Induced pluripotent stem cells from spermatogonial stem cells. In Cell Sources for iPSCs (pp. 15–35). Elsevier. https://doi.org/10.1016/B978-0-12-822135-8.00001-X
Khazaei, M., Ahuja, C. S., & Fehlings, M. G. (2017). Induced pluripotent stem cells for traumatic spinal cord injury. Frontiers in Cell and Developmental Biology, 4(JAN), 1–9. https://doi.org/10.3389/fcell.2016.00152
Streckfuss-Bömeke, K., Wolf, F., Azizian, A., Stauske, M., Tiburcy, M., Wagner, S., & Guan, K. (2013). Comparative study of human-induced pluripotent stem cells derived from bone marrow cells, hair keratinocytes, and skin fibroblasts. European Heart Journal, 34(33), 2618–2629. https://doi.org/10.1093/eurheartj/ehs203
Rohani, L., Johnson, A. A., Arnold, A., & Stolzing, A. (2014). The aging signature: a hallmark of induced pluripotent stem cells? Aging Cell, 13(1), 2–7. https://doi.org/10.1111/acel.12182
Diecke, S., Lu, J., Lee, J., Termglinchan, V., Kooreman, N. G., Burridge, P. W., & Wu, J. C. (2015). Novel codon-optimized mini-intronic plasmid for efficient, inexpensive and xeno-free induction of pluripotency. Scientific Reports, 5(1), 8081. https://doi.org/10.1038/srep08081
Driskell, R. R., & Watt, F. M. (2015). Understanding fibroblast heterogeneity in the skin. Trends in Cell Biology, 25(2), 92–99. https://doi.org/10.1016/j.tcb.2014.10.001
Eminli, S., Utikal, J., Arnold, K., Jaenisch, R., & Hochedlinger, K. (2008). Reprogramming of Neural Progenitor Cells into Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2 Expression. Stem Cells, 26(10), 2467–2474. https://doi.org/10.1634/stemcells.2008-0317
Li, R., Liang, J., Ni, S., Zhou, T., Qing, X., Li, H., & Pei, D. (2010). A mesenchymal-to-Epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell, 7(1), 51–63. https://doi.org/10.1016/j.stem.2010.04.014
Samavarchi-Tehrani, P., Golipour, A., David, L., Sung, H., Beyer, T. A., Datti, A., & Wrana, J. L. (2010). Functional Genomics Reveals a BMP-Driven Mesenchymal-to-Epithelial Transition in the Initiation of Somatic Cell Reprogramming. Cell Stem Cell, 7(1), 64–77. https://doi.org/10.1016/j.stem.2010.04.015
Gore, A., Li, Z., Fung, H.-L., Young, J. E., Agarwal, S., Antosiewicz-Bourget, J., & Zhang, K. (2011). Somatic coding mutations in human induced pluripotent stem cells. Nature, 471(7336), 63–67. https://doi.org/10.1038/nature09805
Abyzov, A., Mariani, J., Palejev, D., Zhang, Y., Haney, M. S., Tomasini, L., & Vaccarino, F. M. (2012). Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature, 492(7429), 438–442. https://doi.org/10.1038/nature11629
Young, M. A., Larson, D. E., Sun, C.-W., George, D. R., Ding, L., Miller, C. A., & Ley, T. J. (2012). Background Mutations in Parental Cells Account for Most of the Genetic Heterogeneity of Induced Pluripotent Stem Cells. Cell Stem Cell, 10(5), 570–582. https://doi.org/10.1016/j.stem.2012.03.002
Zhang, Y., Hu, W., Ma, K., Zhang, C., & Fu, X. (2019). Reprogramming of Keratinocytes as Donor or Target Cells Holds Great Promise for Cell Therapy and Regenerative Medicine. Stem Cell Reviews and Reports, 15(5), 680–689. https://doi.org/10.1007/s12015-019-09900-8
Fuchs, E. (2007). Scratching the surface of skin development. Nature, 445(7130), 834–842. https://doi.org/10.1038/nature05659
Aasen, T., & Belmonte, J. C. I. (2010). Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nature Protocols, 5(2), 371–382. https://doi.org/10.1038/nprot.2009.241
Alonso, L., & Fuchs, E. (2006). The hair cycle. Journal of Cell Science, 119(3), 391–393. https://doi.org/10.1242/jcs02793
Hung, S. S. C., Pébay, A., & Wong, R. C. B. (2015). Generation of integration-free human induced pluripotent stem cells using hair-derived keratinocytes. Journal of Visualized Experiments, 2015(102), 1–6. https://doi.org/10.3791/53174
Klingenstein, S., Klingenstein, M., Kleger, A., & Liebau, S. (2020). From Hair to iPSCs—A Guide on How to Reprogram Keratinocytes and Why. Current Protocols in Stem Cell Biology, 55(1). https://doi.org/10.1002/cpsc.121
Elsholz, F., Harteneck, C., Muller, W., & Friedland, K. (2014). Calcium - A central regulator of keratinocyte differentiation in health and disease. European Journal of Dermatology, 24(6), 650–661. https://doi.org/10.1684/ejd.2014.2452
Aasen, T., Raya, A., Barrero, M. J., Garreta, E., Consiglio, A., Gonzalez, F., & Belmonte, J. C. I. (2008). Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nature Biotechnology, 26(11), 1276–1284. https://doi.org/10.1038/nbt.1503
Re, S., Dogan, A. A., Ben-Shachar, D., Berger, G., Werling, A. M., Walitza, S., & Grünblatt, E. (2018). Improved generation of induced pluripotent stem cells from hair derived keratinocytes – A tool to study neurodevelopmental disorders as ADHD. Frontiers in Cellular Neuroscience, 12(September). https://doi.org/10.3389/fncel.2018.00321
Piao, Y., Hung, S.S.-C., Lim, S. Y., Wong, R.C.-B., & Ko, M. S. H. (2014). Efficient Generation of Integration-Free Human Induced Pluripotent Stem Cells From Keratinocytes by Simple Transfection of Episomal Vectors. STEM CELLS Translational Medicine, 3(7), 787–791. https://doi.org/10.5966/sctm.2013-0036
Lim, S. J., Ho, S. C., Mok, P. L., Tan, K. L., Ong, A. H. K., & Gan, S. C. (2016). Induced pluripotent stem cells from human hair follicle keratinocytes as a potential source for in vitro hair follicle cloning. PeerJ, 2016(11), 1–17. https://doi.org/10.7717/peerj.2695
Giorgetti, A., Montserrat, N., Aasen, T., Gonzalez, F., Rodríguez-Pizà, I., Vassena, R., & Belmonte, J. C. I. (2009). Generation of Induced Pluripotent Stem Cells from Human Cord Blood Using OCT4 and SOX2. Cell Stem Cell, 5(4), 353–357. https://doi.org/10.1016/j.stem.2009.09.008
Maherali, N., Ahfeldt, T., Rigamonti, A., Utikal, J., Cowan, C., & Hochedlinger, K. (2008). A High-Efficiency System for the Generation and Study of Human Induced Pluripotent Stem Cells. Cell Stem Cell, 3(3), 340–345. https://doi.org/10.1016/j.stem.2008.08.003
Li, W., Zhou, H., Abujarour, R., Zhu, S., Joo, J. Y., Lin, T., & Ding, S. (2009). Generation of Human Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2. Stem Cells, N/A-N/A. https://doi.org/10.1002/stem.240
Novak, A., Shtrichman, R., Germanguz, I., Segev, H., Zeevi-Levin, N., Fishman, B., & Itskovitz-Eldor, J. (2010). Enhanced Reprogramming and Cardiac Differentiation of Human Keratinocytes Derived from Plucked Hair Follicles, Using a Single Excisable Lentivirus. Cellular Reprogramming, 12(6), 665–678. https://doi.org/10.1089/cell.2010.0027
Sommer, C. A., & Mostoslavsky, G. (2013). The evolving field of induced pluripotency: Recent progress and future challenges. Journal of Cellular Physiology, 228(2), 267–275. https://doi.org/10.1002/jcp.24155
Brouwer, M., Zhou, H., & Nadif Kasri, N. (2016). Choices for Induction of Pluripotency: Recent Developments in Human Induced Pluripotent Stem Cell Reprogramming Strategies. Stem Cell Reviews and Reports, 12(1), 54–72. https://doi.org/10.1007/s12015-015-9622-8
Kadari, A., Lu, M., Li, M., Sekaran, T., Thummer, R. P., Guyette, N., & Edenhofer, F. (2014). Excision of viral reprogramming cassettes by Cre protein transduction enables rapid, robust and efficient derivation of transgene-free human induced pluripotent stem cells. Stem Cell Research & Therapy, 5(2), 47. https://doi.org/10.1186/scrt435
Sułkowski, M., Konieczny, P., Chlebanowska, P., & Majka, M. (2018). Introduction of exogenous HSV-TK suicide gene increases safety of keratinocyte-derived induced pluripotent stem cells by providing genetic “emergency exit” switch. International Journal of Molecular Sciences, 19(1), 1–16. https://doi.org/10.3390/ijms19010197
Li, C., Ding, L., Sun, C. W., Wu, L. C., Zhou, D., Pawlik, K. M., & Townes, T. M. (2016). Novel HDAd/EBV Reprogramming Vector and Highly Efficient Ad/CRISPR-Cas Sickle Cell Disease Gene Correction. Scientific Reports, 6(July), 1–10. https://doi.org/10.1038/srep30422
Peters, A., & Zambidis, E. T. (2011). Generation of Nonviral Integration-Free Induced Pluripotent Stem Cells from Plucked Human Hair Follicles, (3), 203–227. https://doi.org/10.1007/978-1-61779-267-0_16
Park, T. S., Huo, J. S., Peters, A., Talbot, C. C., Verma, K., Zimmerlin, L., Zambidis, E. T. (2012). Growth factor-activated stem cell circuits and stromal signals cooperatively accelerate non-integrated iPSC reprogramming of human myeloid progenitors. PLoS One, 7(8). https://doi.org/10.1371/journal.pone.0042838
Li, D., Wang, L., Hou, J., Shen, Q., Chen, Q., Wang, X., & Pan, G. (2016). Optimized Approaches for Generation of Integration-free iPSCs from Human Urine-Derived Cells with Small Molecules and Autologous Feeder. Stem Cell Reports, 6(5), 717–728. https://doi.org/10.1016/j.stemcr.2016.04.001
Linta, L., Stockmann, M., Kleinhans, K. N., Böckers, A., Storch, A., Zaehres, H., & Liebau, S. (2012). Rat embryonic fibroblasts improve reprogramming of human keratinocytes into induced pluripotent stem cells. Stem Cells and Development, 21(6), 965–976. https://doi.org/10.1089/scd.2011.0026
Llames, S., García-Pérez, E., Meana, Á., Larcher, F., & Del Río, M. (2015). Feeder Layer Cell Actions and Applications. Tissue Engineering - Part B: Reviews, 21(4), 345–353. https://doi.org/10.1089/ten.teb.2014.0547
Ohmine, S., Dietz, A. B., Deeds, M. C., Hartjes, K. A., Miller, D. R., Thatava, T., & Ikeda, Y. (2011). Induced pluripotent stem cells from GMP-grade hematopoietic progenitor cells and mononuclear myeloid cells. Stem Cell Research and Therapy, 2(6), 46. https://doi.org/10.1186/scrt87
Lowry, W. E., Richter, L., Yachechko, R., Pyle, A. D., Tchieu, J., Sridharan, R., & Plath, K. (2008). Generation of human induced pluripotent stem cells from dermal fibroblasts. Proceedings of the National Academy of Sciences, 105(8), 2883–2888. https://doi.org/10.1073/pnas.0711983105
Nefzger, C. M., Rossello, F. J., Chen, J., Liu, X., Knaupp, A. S., Firas, J., & Polo, J. M. (2017). Cell Type of Origin Dictates the Route to Pluripotency. Cell Reports, 21(10), 2649–2660. https://doi.org/10.1016/j.celrep.2017.11.029
Mikkelsen, T. S., Hanna, J., Zhang, X., Ku, M., Wernig, M., Schorderet, P., & Meissner, A. (2008). Dissecting direct reprogramming through integrative genomic analysis. Nature, 454(7200), 49–55. https://doi.org/10.1038/nature07056
Barrero, M. J., Berdasco, M., Paramonov, I., Bilic, J., Vitaloni, M., Esteller, M., & Belmonte, J. C. I. (2012). DNA hypermethylation in somatic cells correlates with higher reprogramming efficiency. Stem Cells, 30(8), 1696–1702. https://doi.org/10.1002/stem.1138
Panopoulos, A. D., Yanes, O., Ruiz, S., Kida, Y. S., Diep, D., Tautenhahn, R., & Belmonte, J. C. I. (2012). The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Research, 22(1), 168–177. https://doi.org/10.1038/cr.2011.177
Khoo, T. S., Jamal, R., Abdul Ghani, N. A., Alauddin, H., Hussin, N. H., & Abdul Murad, N. A. (2020). Retention of Somatic Memory Associated with Cell Identity, Age and Metabolism in Induced Pluripotent Stem (iPS) Cells Reprogramming. Stem Cell Reviews and Reports, 16(2), 251–261. https://doi.org/10.1007/s12015-020-09956-x
Kawamura, T., Suzuki, J., Wang, Y. V., Menendez, S., Morera, L. B., Raya, A., & Belmonte, J. C. I. (2009). Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature, 460(7259), 1140–1144. https://doi.org/10.1038/nature08311
Li, H., Collado, M., Villasante, A., Strati, K., Ortega, S., Cãamero, M., & Serrano, M. (2009). The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature, 460(7259), 1136–1139. https://doi.org/10.1038/nature08290
Ruiz, S., Panopoulos, A. D., Herrerías, A., Bissig, K. D., Lutz, M., Berggren, W. T., & Izpisua Belmonte, J. C. (2011). A high proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cell identity. Current Biology, 21(1), 45–52. https://doi.org/10.1016/j.cub.2010.11.049
Utikal, J., Polo, J. M., Stadtfeld, M., Maherali, N., Kulalert, W., Walsh, R. M., & Hochedlinger, K. (2009). Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature, 460(7259), 1145–1148. https://doi.org/10.1038/nature08285
Weber, J., Weber, M., Steinle, H., Schlensak, C., Wendel, H. P., & Avci-Adali, M. (2021). An alternative in vivo model to evaluate pluripotency of patient-specific iPSCs. ALTEX. https://doi.org/10.14573/altex.2005221
Warren, L., Manos, P. D., Ahfeldt, T., Loh, Y.-H., Li, H., Lau, F., & Rossi, D. J. (2010). Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell, 7(5), 618–630. https://doi.org/10.1016/j.stem.2010.08.012
Hohwieler, M., Renz, S., Liebau, S., Lin, Q., Lechel, A., Klaus, J., & Kleger, A. (2016). “Miniguts” from plucked human hair meet Crohn’s disease. Zeitschrift für Gastroenterologie, 54(08), 748–759. https://doi.org/10.1055/s-0042-105520
Kim, K., Zhao, R., Doi, A., Ng, K., Unternaehrer, J., Cahan, P., & Daley, G. Q. (2011). Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nature Biotechnology, 29(12), 1117–1119. https://doi.org/10.1038/nbt.2052
Boonkaew, B., Tapeng, L., Netsrithong, R., Vatanashevanopakorn, C., Pattanapanyasat, K., & Wattanapanitch, M. (2018). Induced pluripotent stem cell line MUSIi006-A derived from hair follicle keratinocytes as a non-invasive somatic cell source. Stem Cell Research, 31(July), 79–82. https://doi.org/10.1016/j.scr.2018.07.007
Nakayama, C., Fujita, Y., Matsumura, W., Ujiie, I., Takashima, S., Shinkuma, S., & Shimizu, H. (2018). The development of induced pluripotent stem cell-derived mesenchymal stem/stromal cells from normal human and RDEB epidermal keratinocytes. Journal of Dermatological Science, 91(3), 301–310. https://doi.org/10.1016/j.jdermsci.2018.06.004
Shrestha, R., Wen, Y.-T., Ding, D.-C., & Tsai, R.-K. (2019). Aberrant hiPSCs-Derived from Human Keratinocytes Differentiates into 3D Retinal Organoids that Acquire Mature Photoreceptors. Cells, 8(1), 36. https://doi.org/10.3390/cells8010036
Chlebanowska, P., Sułkowski, M., Skrzypek, K., Tejchman, A., Muszyńska, A., Noroozi, R., & Majka, M. (2020). Origin of the induced pluripotent stem cells affects their differentiation into dopaminergic neurons. International Journal of Molecular Sciences, 21(16), 1–23. https://doi.org/10.3390/ijms21165705
Fleischer, A., Lorenzo, I. M., Palomino, E., Aasen, T., Gómez, F., Servera, M., & Bachiller, D. (2018). Generation of two induced pluripotent stem cell (iPSC) lines from p. F508del Cystic Fibrosis patients. Stem Cell Research, 29, 1–5. https://doi.org/10.1016/j.scr.2018.03.004
Kolundzic, N., Khurana, P., Devito, L., Donne, M., Hobbs, C., Jeriha, J., Ilic, D. (2019). Induced pluripotent stem cell line heterozygous for p.R2447X mutation in filaggrin: KCLi002-A. Stem Cell Research, 38 (March), 101462. https://doi.org/10.1016/j.scr.2019.101462
Zhou, T., Benda, C., Duzinger, S., Huang, Y., Li, X., Li, Y., & Esteban, M. A. (2011). Generation of Induced Pluripotent Stem Cells from Urine. Journal of the American Society of Nephrology, 22(7), 1221–1228. https://doi.org/10.1681/ASN.2011010106
Benda, C., Zhou, T., Wang, X., Tian, W., Grillari, J., Tse, H. F., Esteban, M. A. (2013). Urine as a source of stem cells. In Advances in Biochemical Engineering/Biotechnology, 129, 19–32. https://doi.org/10.1007/10_2012_157
Rahmoune, H., Thompson, P. W., Ward, J. M., Smith, C. D., Hong, G., & Brown, J. (2005). Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes, 54(12), 3427–3434. https://doi.org/10.2337/diabetes.54.12.3427
Guan, X., Mack, D. L., Moreno, C. M., Strande, J. L., Mathieu, J., Shi, Y., & Childers, M. K. (2014). Dystrophin-deficient cardiomyocytes derived from human urine: New biologic reagents for drug discovery. Stem Cell Research, 12(2), 467–480. https://doi.org/10.1016/j.scr.2013.12.004
Drozd, A. M., Walczak, M. P., Piaskowski, S., Stoczynska-Fidelus, E., Rieske, P., & Grzela, D. P. (2015). Generation of human iPSCs from cells of fibroblastic and epithelial origin by means of the oriP/EBNA-1 episomal reprogramming system. Stem Cell Research and Therapy, 6(1), 1–17. https://doi.org/10.1186/s13287-015-0112-3
Bharadwaj, S., Liu, G., Shi, Y., Markert, C., Andersson, K. E., Atala, A., & Zhang, Y. (2011). Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Engineering - Part A, 17(15–16), 2123–2132. https://doi.org/10.1089/ten.tea.2010.0637
Lang, R., Liu, G., Shi, Y., Bharadwaj, S., Leng, X., Zhou, X., & Zhang, Y. (2013). Self-Renewal and Differentiation Capacity of Urine-Derived Stem Cells after Urine Preservation for 24 Hours. PLoS ONE, 8(1), e53980. https://doi.org/10.1371/journal.pone.0053980
Sutherland, G. R., & Bain, A. D. (1972). Culture of Cells from the Urine of Newborn Children. Nature, 239(5369), 231–231. https://doi.org/10.1038/239231a0
Xue, Y., Cai, X., Wang, L., Liao, B., Zhang, H., Shan, Y., Pan, G. (2013). Generating a Non-Integrating Human Induced Pluripotent Stem Cell Bank from Urine-Derived Cells. PLoS One, 8(8). https://doi.org/10.1371/journal.pone.0070573
Sauer, V., Tchaikovskaya, T., Wang, X., Li, Y., Zhang, W., Tar, K., & Roy-Chowdhury, J. (2016). Human urinary epithelial cells as a source of engraftable hepatocyte-like cells using stem cell technology. Cell Transplantation, 25(12), 2221–2243. https://doi.org/10.3727/096368916X692014
Mulder, J., Sharmin, S., Chow, T., Rodrigues, D. C., Hildebrandt, M. R., D’Cruz, R., & Rosenblum, N. D. (2020). Generation of infant- and pediatric-derived urinary induced pluripotent stem cells competent to form kidney organoids. Pediatric Research, 87(4), 647–655. https://doi.org/10.1038/s41390-019-0618-y
Afzal, M. Z., & Strande, J. L. (2015). Generation of induced pluripotent stem cells from muscular dystrophy patients: Efficient integration-free reprogramming of urine derived cells. Journal of Visualized Experiments, 95, 1–8. https://doi.org/10.3791/52032
Gaignerie, A., Lefort, N., Rousselle, M., Forest-Choquet, V., Flippe, L., Francois-Campion, V., & David, L. (2018). Urine-derived cells provide a readily accessible cell type for feeder-free mRNA reprogramming. Scientific Reports, 8(1), 2–11. https://doi.org/10.1038/s41598-018-32645-2
Steinle, H., Weber, M., Behring, A., Mau-Holzmann, U., von Ohle, C., Popov, A. F., & Avci-Adali, M. (2019). Reprogramming of Urine-Derived Renal Epithelial Cells into iPSCs Using srRNA and Consecutive Differentiation into Beating Cardiomyocytes. Molecular Therapy - Nucleic Acids, 17 (September), 907–921. https://doi.org/10.1016/j.omtn.2019.07.016
Bouma, M. J., Arendzen, C. H., Mummery, C. L., Mikkers, H., & Freund, C. (2020). Reprogramming Urine-Derived Cells using Commercially Available Self-Replicative RNA and a Single Electroporation. Current Protocols in Stem Cell Biology, 55(1), 1–17. https://doi.org/10.1002/cpsc.124
Lee, K.-I., Kim, H.-T., & Hwang, D.-Y. (2014). Footprint- and xeno-free human iPSCs derived from urine cells using extracellular matrix-based culture conditions. Biomaterials, 35(29), 8330–8338. https://doi.org/10.1016/j.biomaterials.2014.05.059
Gattazzo, F., Urciuolo, A., & Bonaldo, P. (2014). Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochimica et Biophysica Acta (BBA) - General Subjects, 1840(8), 2506–2519. https://doi.org/10.1016/j.bbagen.2014.01.010
Sun, W., Zhang, S., Zhou, T., Shan, Y., Gao, F., Zhang, Y., & Zhang, X. (2020). Human Urinal Cell Reprogramming: Synthetic 3D Peptide Hydrogels Enhance Induced Pluripotent Stem Cell Population Homogeneity. ACS Biomaterials Science and Engineering, 6(11), 6263–6275. https://doi.org/10.1021/acsbiomaterials.0c00667
Shi, L., Cui, Y., Luan, J., Zhou, X., & Han, J. (2016). Urine-derived induced pluripotent stem cells as a modeling tool to study rare human diseases. Intractable and Rare Diseases Research, 5(3), 192–201. https://doi.org/10.5582/irdr.2016.01062
Pioner, J. M., Guan, X., Klaiman, J. M., Racca, A. W., Pabon, L., Muskheli, V., & Regnier, M. (2020). Absence of full-length dystrophin impairs normal maturation and contraction of cardiomyocytes derived from human-induced pluripotent stem cells. Cardiovascular Research, 116(2), 368–382. https://doi.org/10.1093/cvr/cvz109
Cao, Y., Xu, J., Wen, J., Ma, X., Liu, F., Li, Y., & Huang, G. (2018). Generation of a Urine-Derived Ips Cell Line from a Patient with a Ventricular Septal Defect and Heart Failure and the Robust Differentiation of These Cells to Cardiomyocytes via Small Molecules. Cellular Physiology and Biochemistry, 50(2), 473–488. https://doi.org/10.1159/000494167
Jia, B., Chen, S., Zhao, Z., Liu, P., Cai, J., Qin, D., & Pan, G. (2014). Modeling of hemophilia A using patient-specific induced pluripotent stem cells derived from urine cells. Life Sciences, 108(1), 22–29. https://doi.org/10.1016/j.lfs.2014.05.004
Tang, L., Wang, H., Dai, B., Wang, X., Zhou, D., Shen, J., & Liang, P. (2020). Human induced pluripotent stem cell-derived cardiomyocytes reveal abnormal TGFβ signaling in type 2 diabetes mellitus. Journal of Molecular and Cellular Cardiology, 142 (April), 53–64. https://doi.org/10.1016/j.yjmcc.2020.03.016
Overeem, A. W., Klappe, K., Parisi, S., Klöters-Planchy, P., Mataković, L., du Teil Espina, M., & van IJzendoorn, S. C. D. . (2019). Pluripotent stem cell-derived bile canaliculi-forming hepatocytes to study genetic liver diseases involving hepatocyte polarity. Journal of Hepatology, 71(2), 344–356. https://doi.org/10.1016/j.jhep.2019.03.031
Chen, Y., Luo, R., Xu, Y., Cai, X., Li, W., Tan, K., & Dai, Y. (2013). Generation of systemic lupus erythematosus-specific induced pluripotent stem cells from urine. Rheumatology International, 33(8), 2127–2134. https://doi.org/10.1007/s00296-013-2704-5
Song, J., Yang, X., Zhou, Y., Chen, L., Zhang, X., Liu, Z., & Li, W. (2019). Dysregulation of neuron differentiation in an autistic savant with exceptional memory. Molecular Brain, 12(1), 1–12. https://doi.org/10.1186/s13041-019-0507-7
Yang, X., Liu, Y., Zhou, T., Zhang, H., Dong, R., Li, Y., Gai, Z. (2019). An induced pluripotent stem cells line (SDQLCHi014-A) derived from urine cells of a patient with ASD and hyperactivity carrying a 303 kb de novo deletion at chr3p26.1 implicating GRM7 gene. Stem Cell Research, 41 (September), 2–5. https://doi.org/10.1016/j.scr.2019.101635
Massa, M. G., Gisevius, B., Hirschberg, S., Hinz, L., Schmidt, M., Gold, R., & Haghikia, A. (2016). Multiple sclerosis patient-specific primary neurons differentiated from urinary renal epithelial cells via induced pluripotent stem cells. PLoS ONE, 11(5), 1–14. https://doi.org/10.1371/journal.pone.0155274
Neumeyer, J., Lin, R. Z., Wang, K., Hong, X., Hua, T., Croteau, S. E., & Melero-Martin, J. M. (2019). Bioengineering hemophilia a-specific microvascular grafts for delivery of full-length factor VIII into the bloodstream. Blood Advances, 3(24), 4166–4176. https://doi.org/10.1182/bloodadvances.2019000848
Cai, J., Zhang, Y., Liu, P., Chen, S., Wu, X., Sun, Y., Pei, D. (2013). Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regeneration, 2(1), 2:6. https://doi.org/10.1186/2045-9769-2-6
Li, G., Xie, B., He, L., Zhou, T., Gao, G., Liu, S., Zhong, X. (2018). Generation of retinal organoids with mature rods and cones from urine-derived human induced pluripotent stem cells. Stem Cells International, 2018. https://doi.org/10.1155/2018/4968658
Liu, Y., Zheng, Y., Li, S., Xue, H., Schmitt, K., Hergenroeder, G. W., & Cao, Q. (2017). Human neural progenitors derived from integration-free iPSCs for SCI therapy. Stem Cell Research, 19, 55–64. https://doi.org/10.1016/j.scr.2017.01.004
Lin, V. J. T., Hu, J., Zolekar, A., Yan, L. J., & Wang, Y. C. (2020). Urine Sample-Derived Cerebral Organoids Suitable for Studying Neurodevelopment and Pharmacological Responses. Frontiers in Cell and Developmental Biology, 8 (May), 1–18. https://doi.org/10.3389/fcell.2020.00304
Wang, L., Wang, L., Huang, W., Su, H., Xue, Y., Su, Z., & Pei, D. (2013). Generation of integration-free neural progenitor cells from cells in human urine. Nature Methods, 10(1), 84–89. https://doi.org/10.1038/nmeth.2283
Rossbach, B., Hildebrand, L., El-Ahmad, L., Stachelscheid, H., Reinke, P., & Kurtz, A. (2016). Generation of a human induced pluripotent stem cell line from urinary cells of a healthy donor using an integration free vector. Stem Cell Research, 16(2), 314–317. https://doi.org/10.1016/j.scr.2015.12.018
Steichen, C., Si-Tayeb, K., Wulkan, F., Crestani, T., Rosas, G., Dariolli, R., Krieger, J. E. (2017). Human Induced Pluripotent Stem (hiPS) Cells from Urine Samples: A Non-Integrative and Feeder-Free Reprogramming Strategy. Current Protocols in Human Genetics, 2017 (January), 21.7.1-21.7.22. https://doi.org/10.1002/cphg.26
Uhm, K. O., Jo, E. H., Go, G. Y., Kim, S. J., Choi, H. Y., Im, Y. S., & Koo, S. K. (2017). Generation of human induced pluripotent stem cells from urinary cells of a healthy donor using a non-integration system. Stem Cell Research, 21, 44–46. https://doi.org/10.1016/j.scr.2017.03.019
Wang, L., Chen, Y., Guan, C., Zhao, Z., Li, Q., Yang, J., & Li, J. (2017). Using low-risk factors to generate non-integrated human induced pluripotent stem cells from urine-derived cells. Stem Cell Research and Therapy, 8(1), 1–13. https://doi.org/10.1186/s13287-017-0698-8
Shi, L., Cui, Y., Zhang, G., Zhou, X., Luan, J., & Han, J. (2020). Establishment of a control induced pluripotent stem cell line SMBCi006-A from a healthy male donor. Stem Cell Research, 49 (September), 102025. https://doi.org/10.1016/j.scr.2020.102025
Hildebrand, L., Rossbach, B., Kühnen, P., Gossen, M., Kurtz, A., Reinke, P., & Stachelscheid, H. (2016). Generation of integration free induced pluripotent stem cells from fibrodysplasia ossificans progressiva (FOP) patients from urine samples. Stem Cell Research, 16(1), 54–58. https://doi.org/10.1016/j.scr.2015.11.017
Sochacki, J., Devalle, S., Reis, M., Mattos, P., & Rehen, S. (2016). Generation of urine iPS cell lines from patients with Attention Deficit Hyperactivity Disorder (ADHD) using a non-integrative method. Stem Cell Research, 17(1), 102–106. https://doi.org/10.1016/j.scr.2016.05.015
Li, S., Zhao, H., Huang, R., He, L., Tian, C., Huang, H., & Li, Z. (2019). Generation of iPSC line (GIBHi001-A) from a patient with autism spectrum disorder. Stem Cell Research, 40 (September), 101571. https://doi.org/10.1016/j.scr.2019.101571
Dong, Y., Peng, T., Wu, W., Tan, D., Liu, X., & Xie, D. (2019). Efficient introduction of an isogenic homozygous mutation to induced pluripotent stem cells from a hereditary hearing loss family using CRISPR/Cas9 and single-stranded donor oligonucleotides. Journal of International Medical Research, 47(4), 1717–1730. https://doi.org/10.1177/0300060519829990
Jouni, M., Si-Tayeb, K., Es-Salah-Lamoureux, Z., Latypova, X., Champon, B., Caillaud, A., & Gaborit, N. (2015). Toward personalized medicine: Using cardiomyocytes differentiated from urine-derived pluripotent stem cells to recapitulate electrophysiological characteristics of type 2 long QT syndrome. Journal of the American Heart Association, 4(9), 1–13. https://doi.org/10.1161/JAHA.115.002159
He, L., Zhao, H., Li, S., Han, X., Chen, Z., Wang, C., & Jiang, H. (2020). Generation of induced pluripotent stem cell line (CSUXHi002-A) from a patient with spinocerebellar ataxia type 1. Stem Cell Research, 45 (April), 101816. https://doi.org/10.1016/j.scr.2020.101816
Higdon, L. E., Lee, K., Tang, Q., & Maltzman, J. S. (2016). Virtual Global Transplant Laboratory Standard Operating Procedures for Blood Collection, PBMC Isolation, and Storage. Transplantation Direct, 2(9), e101. https://doi.org/10.1097/txd.0000000000000613
Hanna, J., Markoulaki, S., Schorderet, P., Carey, B. W., Beard, C., Wernig, M., & Jaenisch, R. (2008). Direct Reprogramming of Terminally Differentiated Mature B Lymphocytes to Pluripotency. Cell, 133(2), 250–264. https://doi.org/10.1016/j.cell.2008.03.028
Brown, M. E., Rondon, E., Rajesh, D., Mack, A., Lewis, R., Feng, X., Nuwaysir, E. F. (2010). Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS One, 5(6). https://doi.org/10.1371/journal.pone.0011373
Loh, Y. H., Hartung, O., Li, H., Guo, C., Sahalie, J. M., Manos, P. D., & Daley, G. Q. (2010). Reprogramming of T cells from human peripheral blood. Cell Stem Cell, 7(1), 15–19. https://doi.org/10.1016/j.stem.2010.06.004
Hong, H., Takahashi, K., Ichisaka, T., Aoi, T., Kanagawa, O., Nakagawa, M., & Yamanaka, S. (2009). Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature, 460(7259), 1132–1135. https://doi.org/10.1038/nature08235
Seki, T., Yuasa, S., Oda, M., Egashira, T., Yae, K., Kusumoto, D., & Fukuda, K. (2010). Generation of induced pluripotent stem cells from human terminally differentiated circulating t cells. Cell Stem Cell, 7(1), 11–14. https://doi.org/10.1016/j.stem.2010.06.003
Choi, S. M., Liu, H., Chaudhari, P., Kim, Y., Cheng, L., Feng, J., & Jang, Y.-Y. (2011). Reprogramming of EBV-immortalized B-lymphocyte cell lines into induced pluripotent stem cells. Blood, 118(7), 1801–1805. https://doi.org/10.1182/blood-2011-03-340620
Rajesh, D., Dickerson, S. J., Yu, J., Brown, M. E., Thomson, J. A., & Seay, N. J. (2011). Human lymphoblastoid B-cell lines reprogrammed to EBV-free induced pluripotent stem cells. Blood, 118(7), 1797–1800. https://doi.org/10.1182/blood-2011-01-332064
Barrett, R., Ornelas, L., Yeager, N., Mandefro, B., Sahabian, A., Lenaeus, L., & Sareen, D. (2014). Reliable Generation of Induced Pluripotent Stem Cells From Human Lymphoblastoid Cell Lines. STEM CELLS Translational Medicine, 3(12), 1429–1434. https://doi.org/10.5966/sctm.2014-0121
Kishino, Y., Seki, T., Fujita, J., Yuasa, S., Tohyama, S., Kunitomi, A., & Fukuda, K. (2014). Derivation of transgene-free human induced pluripotent stem cells from human peripheral T cells in defined culture conditions. PLoS ONE, 9(5), 1–8. https://doi.org/10.1371/journal.pone.0097397
Muñoz-López, Á., Van Roon, E. H. J., Romero-Moya, D., López-Millan, B., Stam, R. W., Colomer, D., & Menendez, P. (2016). Cellular ontogeny and hierarchy influence the reprogramming efficiency of human B cells into induced pluripotent stem cells. Stem Cells, 34(3), 581–587. https://doi.org/10.1002/stem.2303
Chou, B.-K., Gu, H., Gao, Y., Dowey, S. N., Wang, Y., Shi, J., & Cheng, L. (2015). A Facile Method to Establish Human Induced Pluripotent Stem Cells From Adult Blood Cells Under Feeder-Free and Xeno-Free Culture Conditions: A Clinically Compliant Approach. STEM CELLS Translational Medicine, 4(4), 320–332. https://doi.org/10.5966/sctm.2014-0214
Serwold, T., Hochedlinger, K., Swindle, J., Hedgpeth, J., Jaenisch, R., & Weissman, I. L. (2010). T-cell receptor-driven lymphomagenesis in mice derived from a reprogrammed T cell. Proceedings of the National Academy of Sciences, 107(44), 18939–18943. https://doi.org/10.1073/pnas.1013230107
Dowey, S. N., Huang, X., Chou, B. K., Ye, Z., & Cheng, L. (2012). Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nature Protocols, 7(11), 2013–2021. https://doi.org/10.1038/nprot.2012.121
Loh, Y. H., Agarwal, S., Park, I. H., Urbach, A., Huo, H., Heffner, G. C., & Daley, G. Q. (2009). Generation of induced pluripotent stem cells from human blood. Blood, 113(22), 5476–5479. https://doi.org/10.1182/blood-2009-02-204800
Ye, Z., Zhan, H., Mali, P., Dowey, S., Williams, D. M., Jang, Y. Y., & Cheng, L. (2009). Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood, 114(27), 5473–5480. https://doi.org/10.1182/blood-2009-04-217406
Merling, R. K., Sweeney, C. L., Choi, U., De Ravin, S. S., Myers, T. G., Otaizo-Carrasquero, F., & Malech, H. L. (2013). Transgene-free iPSCs generated from small volume peripheral blood nonmobilized CD34+ cells. Blood, 121(14), e98–e107. https://doi.org/10.1182/blood-2012-03-420273
Mack, A. A., Kroboth, S., Rajesh, D., & Wang, W. B. (2011). Generation of Induced Pluripotent Stem Cells from CD34+ Cells across Blood Drawn from Multiple Donors with Non-Integrating Episomal Vectors. PLoS ONE, 6(11), e27956. https://doi.org/10.1371/journal.pone.0027956
de Leeuw, V. C., van Oostrom, C. T. M., Imholz, S., Piersma, A. H., Hessel, E. V. S., & Dollé, M. E. T. (2020). Going Back and Forth: Episomal Vector Reprogramming of Peripheral Blood Mononuclear Cells to Induced Pluripotent Stem Cells and Subsequent Differentiation into Cardiomyocytes and Neuron-Astrocyte Co-cultures. Cellular Reprogramming, 22(6), 300–310. https://doi.org/10.1089/cell.2020.0040
Sohn, S. K., Kim, J. G., Seo, K. W., Chae, Y. S., Jung, J. T., Suh, J. S., & Lee, K. B. (2002). GM-CSF-based mobilization effect in normal healthy donors for allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplantation, 30(2), 81–86. https://doi.org/10.1038/sj.bmt.1703598
Cashen, A. F., Lazarus, H. M., & Devine, S. M. (2007). Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF? Bone Marrow Transplantation, 39(10), 577–588. https://doi.org/10.1038/sj.bmt.1705616
Szilvassy, S. J., Meyerrose, T. E., Ragland, P. L., & Grimes, B. (2001). Differential homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow, mobilized peripheral blood, and fetal liver. Blood, 98(7), 2108–2115. https://doi.org/10.1182/blood.V98.7.2108
Nagler, A., Korenstein-Ilan, A., Amiel, A., & Avivi, L. (2004). Granulocyte colony-stimulating factor generates epigenetic and genetic alterations in lymphocytes of normal volunteer donors of stem cells. Experimental Hematology, 32(1), 122–130. https://doi.org/10.1016/j.exphem.2003.09.007
Hernández, J. M., Castilla, C., Gutiérrez, N. C., Isidro, I. M., Delgado, M., & de las Rivas, J., San Miguel, J. F. . (2005). Mobilisation with G-CSF in healthy donors promotes a high but temporal deregulation of genes. Leukemia, 19(6), 1088–1091. https://doi.org/10.1038/sj.leu.2403753
Kunisato, A., Wakatsuki, M., Shinba, H., Ota, T., Ishida, I., & Nagao, K. (2011). Direct generation of induced pluripotent stem cells from human nonmobilized blood. Stem Cells and Development, 20(1), 159–168. https://doi.org/10.1089/scd.2010.0063
Zheng, W., Wang, Y., Chang, T., Huang, H., & Yee, J.-K. (2013). Significant differences in genotoxicity induced by retrovirus integration in human T cells and induced pluripotent stem cells. Gene, 519(1), 142–149. https://doi.org/10.1016/j.gene.2013.01.009
Potirat, P., Wattanapanitch, M., Kheolamai, P., & Issaragrisil, S. (2018). Establishment of a human iPSC line (MUSIi007-A) from peripheral blood of normal individual using Sendai viral vectors. Stem Cell Research, 32 (July), 43–46. https://doi.org/10.1016/j.scr.2018.08.014
Okumura, T., Horie, Y., Lai, C. Y., Lin, H. T., Shoda, H., Natsumoto, B., & Otsu, M. (2019). Robust and highly efficient hiPSC generation from patient non-mobilized peripheral blood-derived CD34+ cells using the auto-erasable Sendai virus vector. Stem Cell Research and Therapy, 10(1), 4–6. https://doi.org/10.1186/s13287-019-1273-2
Vlahos, K., Sourris, K., Mayberry, R., McDonald, P., Bruveris, F. F., Schiesser, J. V., Elefanty, A. G. (2019). Generation of iPSC lines from peripheral blood mononuclear cells from 5 healthy adults. Stem Cell Research, 34 (November 2018), 101380. https://doi.org/10.1016/j.scr.2018.101380
Hamada, A., Akagi, E., Obayashi, F., Yamasaki, S., Koizumi, K., Ohtaka, M., & Okamoto, T. (2020). Induction of Noonan syndrome-specific human-induced pluripotent stem cells under serum-, feeder-, and integration-free conditions. Vitro Cellular and Developmental Biology - Animal, 56(10), 888–895. https://doi.org/10.1007/s11626-020-00515-9
Ye, L., Muench, M. O., Fusaki, N., Beyer, A. I., Wang, J., Qi, Z., & Kan, Y. W. (2013). Blood Cell-Derived Induced Pluripotent Stem Cells Free of Reprogramming Factors Generated by Sendai Viral Vectors. STEM CELLS Translational Medicine, 2(8), 558–566. https://doi.org/10.5966/sctm.2013-0006
Tan, H.-K., Toh, C.-X.D., Ma, D., Yang, B., Liu, T. M., Lu, J., & Loh, Y.-H. (2014). Human Finger-Prick Induced Pluripotent Stem Cells Facilitate the Development of Stem Cell Banking. STEM CELLS Translational Medicine, 3(5), 586–598. https://doi.org/10.5966/sctm.2013-0195
Su, R. J., Neises, A., & Zhang, X.-B. (2016). Generation of iPS Cells from Human Peripheral Blood Mononuclear Cells Using Episomal Vectors. In Methods in Molecular Biology (pp. 57–69). https://doi.org/10.1007/7651_2014_139
Sharma, A., Mücke, M., & Seidman, C. E. (2018). Human Induced Pluripotent Stem Cell Production and Expansion from Blood using a Non‐Integrating Viral Reprogramming Vector. Current Protocols in Molecular Biology, 122(1). https://doi.org/10.1002/cpmb.58
Okita, K., Yamakawa, T., Matsumura, Y., Sato, Y., Amano, N., Watanabe, A., & Yamanaka, S. (2013). An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells, 31(3), 458–466. https://doi.org/10.1002/stem.1293
Su, R. J., Baylink, D. J., Neises, A., Kiroyan, J. B., Meng, X., Payne, K. J., & Zhang, X. B. (2013). Efficient Generation of Integration-Free iPS Cells from Human Adult Peripheral Blood Using BCL-XL Together with Yamanaka Factors. PLoS ONE, 8(5), 1–12. https://doi.org/10.1371/journal.pone.0064496
Nakagawa, M., Taniguchi, Y., Senda, S., Takizawa, N., Ichisaka, T., Asano, K., & Yamanaka, S. (2014). A novel efficient feeder-Free culture system for the derivation of human induced pluripotent stem cells. Scientific Reports, 4, 1–7. https://doi.org/10.1038/srep03594
Agu, C. A., Soares, F. A. C., Alderton, A., Patel, M., Ansari, R., Patel, S., & Kirton, C. M. (2015). Successful generation of human induced pluripotent stem cell lines from blood samples held at room temperature for up to 48 hr. Stem Cell Reports, 5(4), 660–671. https://doi.org/10.1016/j.stemcr.2015.08.012
Liu, J., Brzeszczynska, J., Samuel, K., Black, J., Palakkan, A., Anderson, R. A., & Ross, J. A. (2015). Efficient episomal reprogramming of blood mononuclear cells and differentiation to hepatocytes with functional drug metabolism. Experimental Cell Research, 338(2), 203–213. https://doi.org/10.1016/j.yexcr.2015.08.004
Zhou, H., Martinez, H., Sun, B., Li, A., Zimmer, M., Katsanis, N., & Chang, S. (2015). Rapid and efficient generation of transgene-free iPSC from a small volume of cryopreserved blood. Stem Cell Reviews and Reports, 11(4), 652–665. https://doi.org/10.1007/s12015-015-9586-8
Sommer, A. G., Rozelle, S. S., Sullivan, S., Mills, J. A., Park, S. M., Smith, B. W., & Mostoslavsky, G. (2012). Generation of human induced pluripotent stem cells from peripheral blood using the STEMCCA lentiviral vector. Journal of visualized experiments : JoVE, 68, 1–5. https://doi.org/10.3791/4327
Chen, I. P., Fukuda, K., Fusaki, N., Iida, A., Hasegawa, M., Lichtler, A., & Reichenberger, E. J. (2013). Induced pluripotent stem cell reprogramming by integration-free sendai virus vectors from peripheral blood of patients with craniometaphyseal dysplasia. Cellular Reprogramming, 15(6), 503–513. https://doi.org/10.1089/cell.2013.0037
Quintana-Bustamante, O., & Segovia, J. C. (2016). Generation of patient-specific induced pluripotent stem cell from peripheral blood mononuclear cells by sendai reprogramming vectors. Methods in Molecular Biology, 1353(17), 1–11. https://doi.org/10.1007/7651_2014_170
Kim, Y., Rim, Y. A., Yi, H., Park, N., Park, S. H., & Ju, J. H. (2016). The Generation of Human Induced Pluripotent Stem Cells from Blood Cells: An Efficient Protocol Using Serial Plating of Reprogrammed Cells by Centrifugation. Stem Cells International, 2016. https://doi.org/10.1155/2016/1329459
Ali, M., Kabir, F., Thomson, J. J., Ma, Y., Qiu, C., Delannoy, M., & Riazuddin, S. A. (2019). Comparative transcriptome analysis of hESC- and iPSC-derived lentoid bodies. Scientific Reports, 9(1), 1–9. https://doi.org/10.1038/s41598-019-54258-z
Isogai, S., Yamamoto, N., Hiramatsu, N., Goto, Y., Hayashi, M., Kondo, M., & Imaizumi, K. (2018). Preparation of induced pluripotent stem cells using human peripheral blood monocytes. Cellular Reprogramming, 20(6), 347–355. https://doi.org/10.1089/cell.2018.0024
Ye, H., & Wang, Q. (2018). Efficient Generation of Non-Integration and Feeder-Free Induced Pluripotent Stem Cells from Human Peripheral Blood Cells by Sendai Virus. Cellular Physiology and Biochemistry, 50(4), 1318–1331. https://doi.org/10.1159/000494589
Guan, J., Liu, X., Zhang, H., Yang, X., Ma, Y., Li, Y., Liu, Y. (2020). Reprogramming of human Peripheral Blood Mononuclear Cell (PBMC) from a Chinese patient suffering Duchenne muscular dystrophy to iPSC line (SDQLCHi007-A) carrying deletion of 49–50 exons in the DMD gene. Stem Cell Research, 42 (September 2019). https://doi.org/10.1016/j.scr.2019.101666
Trokovic, R., Weltner, J., Nishimura, K., Ohtaka, M., Nakanishi, M., Salomaa, V., & Kyttälä, A. (2014). Advanced Feeder-Free Generation of Induced Pluripotent Stem Cells Directly From Blood Cells. STEM CELLS Translational Medicine, 3(12), 1402–1409. https://doi.org/10.5966/sctm.2014-0113
Chou, B. K., Mali, P., Huang, X., Ye, Z., Dowey, S. N., Resar, L. M. S., & Cheng, L. (2011). Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Research, 21(3), 518–529. https://doi.org/10.1038/cr.2011.12
Morales Pantoja, I. E., Smith, M. D., Rajbhandari, L., Cheng, L., Gao, Y., Mahairaki, V., & Whartenby, K. A. (2020). IPSCs from people with MS can differentiate into oligodendrocytes in a homeostatic but not an inflammatory milieu. PLoS ONE, 15(6), 1–19. https://doi.org/10.1371/journal.pone.0233980
Wen, W., Zhang, J. P., Xu, J., Su, R. J., Neises, A., Ji, G. Z., & Zhang, X. B. (2016). Enhanced generation of integration-free iPSCs from human adult peripheral blood mononuclear cells with an optimal combination of episomal vectors. Stem Cell Reports, 6(6), 873–884. https://doi.org/10.1016/j.stemcr.2016.04.005
Ustyantseva, E. I., Medvedev, S. P., Vetchinova, A. S., Illarioshkin, S. N., Leonov, S. V., & Zakian, S. M. (2020). Generation of an induced pluripotent stem cell line, ICGi014-A, by reprogramming peripheral blood mononuclear cells from a patient with homozygous D90A mutation in SOD1 causing Amyotrophic lateral sclerosis. Stem Cell Research, 42 (September 2019), 101675. https://doi.org/10.1016/j.scr.2019.101675
Staerk, J., Dawlaty, M. M., Gao, Q., Maetzel, D., Hanna, J., Sommer, C. A., & Jaenisch, R. (2010). Reprogramming of Human Peripheral Blood Cells to Induced Pluripotent Stem Cells. Cell Stem Cell, 7(1), 20–24. https://doi.org/10.1016/j.stem.2010.06.002
Riedel, M., Jou, C. J., Lai, S., Lux, R. L., Moreno, A. P., Spitzer, K. W., & Benjamin, I. J. (2014). Functional and pharmacological analysis of cardiomyocytes differentiated from human peripheral blood mononuclear-derived pluripotent stem cells. Stem Cell Reports, 3(1), 131–141. https://doi.org/10.1016/j.stemcr.2014.04.017
Fuerstenau-Sharp, M., Zimmermann, M. E., Stark, K., Jentsch, N., Klingenstein, M., Drzymalski, M., & Hengstenberg, C. (2015). Generation of highly purified human cardiomyocytes from peripheral blood mononuclear cell-derived induced pluripotent stem cells. PLoS ONE, 10(5), 1–21. https://doi.org/10.1371/journal.pone.0126596
Hanatani, T., & Takasu, N. (2021). CiRA iPSC seed stocks (CiRA’s iPSC Stock Project). Stem Cell Research, 50 (April 2019), 102033. https://doi.org/10.1016/j.scr.2020.102033
Churko, J. M., Burridge, P. W., & Wu, J. C. (2013). Generation of human iPSCs from human peripheral blood mononuclear cells using non-integrative sendai virus in chemically defined conditions. In Methods in Molecular Biology (Vol. 1036, pp. 81–88). https://doi.org/10.1007/978-1-62703-511-8_7
Varga, E., Hansen, M., Wüst, T., von Lindern, M., & van den Akker, E. (2017). Generation of human erythroblast-derived iPSC line using episomal reprogramming system. Stem Cell Research, 25, 30–33. https://doi.org/10.1016/j.scr.2017.10.001
Varga, E., Nemes, C., Bock, I., Varga, N., Fehér, A., Dinnyés, A., & Kobolák, J. (2016). Generation of Mucopolysaccharidosis type II (MPS II) human induced pluripotent stem cell (iPSC) line from a 1-year-old male with pathogenic IDS mutation. Stem Cell Research, 17(3), 482–484. https://doi.org/10.1016/j.scr.2016.09.033
Lopez-Onieva, L., Montes, R., Lamolda, M., Romero, T., Ayllon, V., Lozano, M. L., & Real, P. J. (2016). Generation of induced pluripotent stem cells (iPSCs) from a Bernard-Soulier syndrome patient carrying a W71R mutation in the GPIX gene. Stem Cell Research, 16(3), 692–695. https://doi.org/10.1016/j.scr.2016.04.013
Marsoner, F., Marcatili, M., Karnavas, T., Bottai, D., D’Agostino, A., Scarone, S., & Conti, L. (2016). Generation and characterization of an induced pluripotent stem cell (iPSC) line from a patient with clozapine-resistant Schizophrenia. Stem Cell Research, 17(3), 661–664. https://doi.org/10.1016/j.scr.2016.11.005
Lee, H.-K., Morin, P., & Xia, W. (2016). Peripheral blood mononuclear cell-converted induced pluripotent stem cells (iPSCs) from an early onset Alzheimer’s patient. Stem Cell Research, 16(2), 213–215. https://doi.org/10.1016/j.scr.2015.12.050
Táncos, Z., Varga, E., Kovács, E., Dinnyés, A., & Kobolák, J. (2016). Establishment of induced pluripotent stem cell (iPSC) line from a 75-year old patient with late onset Alzheimer’s disease (LOAD). Stem Cell Research, 17(1), 81–83. https://doi.org/10.1016/j.scr.2016.05.013
Griscelli, F., Oudrirhi, N., Feraud, O., Divers, D., Portier, L., Turhan, A. G., & Bennaceur Griscelli, A. (2017). Generation of induced pluripotent stem cell (iPSC) line from a patient with triple negative breast cancer with hereditary exon 17 deletion of BRCA1 gene. Stem Cell Research, 24, 135–138. https://doi.org/10.1016/j.scr.2017.09.003
Zhang, S., Liu, L., Hu, Y., Lv, Z., Li, Q., Gong, W., & Wu, H. (2017). Derivation of human induced pluripotent stem cell (iPSC) line from a 79 year old sporadic male Parkinson’s disease patient. Stem Cell Research, 19, 43–45. https://doi.org/10.1016/j.scr.2016.12.025
Bhargava, N., Jaitly, S., Goswami, S. G., Jain, S., Chakraborty, D., & Ramalingam, S. (2019). Generation and characterization of induced pluripotent stem cell line (IGIBi001-A) from a sickle cell anemia patient with homozygous β-globin mutation. Stem Cell Research, 39 (June), 101484. https://doi.org/10.1016/j.scr.2019.101484
Bozaoglu, K., Gao, Y., Stanley, E., Fanjul-Fernández, M., Brown, N. J., Pope, K., & Lockhart, P. J. (2019). Generation of seven iPSC lines from peripheral blood mononuclear cells suitable to investigate Autism Spectrum Disorder. Stem Cell Research, 39 (June), 101516. https://doi.org/10.1016/j.scr.2019.101516
Gatinois, V., Desprat, R., Becker, F., Pichard, L., Bernex, F., Corsini, C., & Lemaitre, J. M. (2019). Reprogramming of Human Peripheral Blood Mononuclear Cell (PBMC) from a patient suffering of a Werner syndrome resulting in iPSC line (REGUi003-A) maintaining a short telomere length. Stem Cell Research, 39 (July), 101515. https://doi.org/10.1016/j.scr.2019.101515
Gubert, F., Vasques, J. F., Cozendey, T. D., Domizi, P., Toledo, M. F., Kasai-Brunswick, T. H., & Mendez-Otero, R. (2019). Generation of four patient-specific pluripotent induced stem cell lines from two Brazilian patients with amyotrophic lateral sclerosis and two healthy subjects. Stem Cell Research, 37 (February), 101448. https://doi.org/10.1016/j.scr.2019.101448
Lamolda, M., Montes, R., Simón, I., Perales, S., Martínez-Navajas, G., Lopez-Onieva, L., Real, P. J. (2019). GENYOi005-A: An induced pluripotent stem cells (iPSCs) line generated from a patient with Familial Platelet Disorder with associated Myeloid Malignancy (FPDMM) carrying a p.Thr196Ala variant. Stem Cell Research, 41 (September), 101603. https://doi.org/10.1016/j.scr.2019.101603
Montes, R., Mollinedo, P., Perales, S., Gonzalez-Lamuño, D., Ramos-Mejía, V., Fernandez-Luna, J. L., & Real, P. J. (2019). GENYOi004-A: An induced pluripotent stem cells (iPSCs) line generated from a patient with autism-related ADNP syndrome carrying a pTyr719* mutation. Stem Cell Research, 37, 101446. https://doi.org/10.1016/j.scr.2019.101446
Piovani, G., Lanzi, G., Ferraro, R. M., Masneri, S., Barisani, C., Savio, G., & Giliani, S. C. (2019). Generation of induced pluripotent stem cells (iPSCs) from patient with Cri du Chat Syndrome. Stem Cell Research, 35 (December 2018), 101393. https://doi.org/10.1016/j.scr.2019.101393
Tong, J., Lee, K. M., Liu, X., Nefzger, C. M., Vijayakumar, P., Hawi, Z., Bellgrove, M. A. (2019). Generation of four iPSC lines from peripheral blood mononuclear cells (PBMCs) of an attention deficit hyperactivity disorder (ADHD) individual and a healthy sibling in an Australia-Caucasian family. Stem Cell Research, 34 (November 2018), 101353. https://doi.org/10.1016/j.scr.2018.11.014
Zhang, Y., Li, A., Huang, C. L. H., Wang, G., & Wang, D. (2019). Generation of induced pluripotent stem cells (iPSCs) from an infant with catecholaminergic polymorphic ventricular tachycardia carrying the double heterozygous mutations A1855D in RyR2 and Q1362H in SCN10A. Stem Cell Research, 39 (December 2018), 101509. https://doi.org/10.1016/j.scr.2019.101509
Zhu, Y.-J., Zhang, S.-J., Wu, X.-H., Lian, T.-Y., He, Y.-Z., Zhang, Z.-J., Jing, Z.-C. (2020). Generation of an induced pluripotent stem cell line (PUMCHi006-A) derived from a patient with pulmonary arterial hypertension carrying heterozygous c.1339 G > A mutation in PTGIS gene. Stem Cell Research, 49, 102088. https://doi.org/10.1016/j.scr.2020.102088
Thakur, P., Bhargava, N., Jaitly, S., Gupta, P., Kumar Bhattacharya, S., Padma, G., & Ramalingam, S. (2021). Establishment and characterization of induced pluripotent stem cell line (IGIBi002-A) from a β-thalassemia patient with IVS1-5 mutation by non-integrating reprogramming approach. Stem Cell Research, 50, 102124. https://doi.org/10.1016/j.scr.2020.102124
Wang, Y., Yu, H., Chen, Y., Li, G., Lei, Y., & Zhao, J. (2018). Derivation of induced pluripotent stem cells TUSMi006 from an 87-year old Chinese Han Alzheimer’s disease patient carrying GRINB and SORL1 mutations. Stem Cell Research, 31 (July), 127–130. https://doi.org/10.1016/j.scr.2018.07.018
Zhang, L., Xu, M., Liu, G., Wu, R., Meng, S., Xiahou, K., & Zhou, W. (2019). Generation of induced pluripotent stem cell line (IPTi001-A) from a 62-year old sporadic Alzheimer’s disease patient with APOE3 (ε3/ε3) genotype. Stem Cell Research, 41 (September), 101589. https://doi.org/10.1016/j.scr.2019.101589
Zhao, Z., Ji, S., Shi, Z., & Liu, H. (2018). Generation of CSi001-A, a transgene-free, induced pluripotent stem cell line derived from a Parkinson Disease (PD) patient. Stem Cell Research, 33 (September), 1–5. https://doi.org/10.1016/j.scr.2018.09.020
Kamath, A., Ternes, S., McGowan, S., & Moy, A. B. (2018). Virus-free and oncogene-free induced pluripotent stem cell reprogramming in cord blood and peripheral blood in patients with lung disease. Regenerative Medicine, 13(8), 899–915. https://doi.org/10.2217/rme-2018-0041
Paredes, B. D., Martins, G. L. S., Azevedo, C. M., de Sampaio, G. L., & A., Nonaka, C. K. V., Silva, K. N. da, Souza, B. S. de F. . (2019). Generation of three control iPS cell lines for sickle cell disease studies by reprogramming erythroblasts from individuals without hemoglobinopathies. Stem Cell Research, 38 (March), 101454. https://doi.org/10.1016/j.scr.2019.101454
Laperle, A. H., Sances, S., Yucer, N., Dardov, V. J., Garcia, V. J., Ho, R., & Svendsen, C. N. (2020). iPSC modeling of young-onset Parkinson’s disease reveals a molecular signature of disease and novel therapeutic candidates. Nature Medicine, 26(2), 289–299. https://doi.org/10.1038/s41591-019-0739-1
Gao, X., Liao, X., Zhang, J., Lin, J., & Tan, M. (2021). Derivation of an induced pluripotent stem cell line (GWCMCi001-A) from PBMCs of a four-year-old male patient with Immunoglobulin A nephropathy. Stem Cell Research, 50, 102123. https://doi.org/10.1016/j.scr.2020.102123
Wang, B., Liu, C., Zhang, H., Gai, Z., & Liu, Y. (2021). An induced pluripotent stem cell line (SDQLCHi033-A) derived from a patient with maple syrup urine disease type Ib carrying a homozygous mutation in BCKDHB gene. Stem Cell Research, 50, 102146. https://doi.org/10.1016/j.scr.2020.102146
Saha, B., Borgohain, M., Dey, C., & Thummer, R. (2018). Annals of Stem Cell Research & Therapy iPS Cell Generation : Current and Future Challenges. Annals of Stem Cell Research & Therapy, 1(2), 2–5.
Dey, C., Narayan, G., Krishna Kumar, H., Borgohain, M., & Lenka, N. (2017). Cell-Penetrating Peptides as a Tool to Deliver Biologically Active Recombinant Proteins to Generate Transgene-Free Induced Pluripotent Stem Cells. Studies on Stem Cells Research and Therapy, 3(1), 006–015. https://doi.org/10.17352/sscrt.000011
Ebrahimi, B. (2015). Reprogramming of adult stem/progenitor cells into iPSCs without reprogramming factors. Journal of Medical Hypotheses and Ideas, 9(2), 99–103. https://doi.org/10.1016/j.jmhi.2015.09.003
Geti, I., Ormiston, M. L., Rouhani, F., Toshner, M., Movassagh, M., Nichols, J., & Morrell, N. W. (2012). A Practical and Efficient Cellular Substrate for the Generation of Induced Pluripotent Stem Cells from Adults: Blood-Derived Endothelial Progenitor Cells. STEM CELLS Translational Medicine, 1(12), 855–865. https://doi.org/10.5966/sctm.2012-0093
Naujok, O., Kaldrack, J., Taivankhuu, T., Jörns, A., & Lenzen, S. (2010). Selective Removal of Undifferentiated Embryonic Stem Cells from Differentiation Cultures Through HSV1 Thymidine Kinase and Ganciclovir Treatment. Stem Cell Reviews and Reports, 6(3), 450–461. https://doi.org/10.1007/s12015-010-9148-z
Ben-David, U., Mayshar, Y., & Benvenisty, N. (2011). Large-Scale Analysis Reveals Acquisition of Lineage-Specific Chromosomal Aberrations in Human Adult Stem Cells. Cell Stem Cell, 9(2), 97–102. https://doi.org/10.1016/j.stem.2011.06.013
Lee, M.-O., Moon, S. H., Jeong, H.-C., Yi, J.-Y., Lee, T.-H., Shim, S. H., & Cha, H.-J. (2013). Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proceedings of the National Academy of Sciences, 110(35), E3281–E3290. https://doi.org/10.1073/pnas.1303669110
Mohseni, R. (2014). Safe Transplantation Of Pluripotent Stem Cell By Preventing Teratoma Formation. Journal of Stem Cell Research & Therapy, 04(06). https://doi.org/10.4172/2157-7633.1000212
Parr, C. J. C., Katayama, S., Miki, K., Kuang, Y., Yoshida, Y., Morizane, A., & Saito, H. (2016). MicroRNA-302 switch to identify and eliminate undifferentiated human pluripotent stem cells. Scientific Reports, 6(1), 32532. https://doi.org/10.1038/srep32532
Kuang, Y., Miki, K., Parr, C. J. C., Hayashi, K., Takei, I., Li, J., & Saito, H. (2017). Efficient, Selective Removal of Human Pluripotent Stem Cells via Ecto-Alkaline Phosphatase-Mediated Aggregation of Synthetic Peptides. Cell Chemical Biology, 24(6), 685-694.e4. https://doi.org/10.1016/j.chembiol.2017.04.010
Sperling., E. L. (2013). Embryonic Stem Cell Therapy – From Bench to Bed. In Pluripotent Stem Cells. InTech. https://doi.org/10.5772/54368
Borgohain, M. P., Narayan, G., Krishna Kumar, H., Dey, C., & Thummer, R. P. (2018). Maximizing Expression and Yield of Human Recombinant Proteins from Bacterial Cell Factories for Biomedical Applications. In P. Kumar, J. K. Patra, & P. Chandra (Eds.), Advances in Microbial Biotechnology (1st Edition, pp. 447–486). New York: Apple Academic Press. https://doi.org/10.1201/9781351248914
Acknowledgements
We thank all the members of the Laboratory for Stem Cell Engineering and Regenerative Medicine (SCERM) for their excellent support. This work was supported by North Eastern Region – Biotechnology Programme Management Cell (NERBPMC), Department of Biotechnology, Government of India (BT/PR16655/NER/95/132/2015), and also by IIT Guwahati Institutional Top-Up on Start-Up Grant.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this manuscript. Data collection and interpretation were performed by all the authors. The first draft of the manuscript was written by Arnab Ray (wrote the section on urine cells) and Jahnavy Madhukar Joshi (wrote the section on PBMCs) and Pradeep Kumar Sundaravadivelu (wrote the section on keratinocytes) and thoroughly cross-checked by Khyati Raina. All the authors commented on the previous versions of the manuscript. All authors read and approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ray, A., Joshi, J.M., Sundaravadivelu, P.K. et al. An Overview on Promising Somatic Cell Sources Utilized for the Efficient Generation of Induced Pluripotent Stem Cells. Stem Cell Rev and Rep 17, 1954–1974 (2021). https://doi.org/10.1007/s12015-021-10200-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10200-3