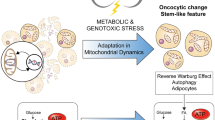
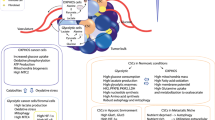
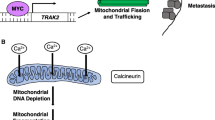
Abstract
Cancer stem cells (CSCs) are rare populations of malignant cells with stem cell-like features of self-renewal, uninterrupted differentiation, tumorigenicity, and resistance to conventional therapeutic agents, and these cells have a decisive role in treatment failure and tumor relapse. The self-renewal potential of CSCs with atypical activation of developmental signaling pathways involves the maintenance of stemness to support cancer progression. The acquisition of stemness in CSCs has been accomplished through genetic and epigenetic rewiring following the metabolic switch. In this context, “metabostemness” denotes the metabolic parameters that essentially govern the epitranscriptional gene reprogramming mechanism to dedifferentiate tumor cells into CSCs. Several metabolites often referred to as oncometabolites can directly remodel chromatin structure and thereby influence the operation of epitranscriptional circuits. This integrated metaboloepigenetic dimension of CSCs favors the differentiated cells to move in dedifferentiated macrostates. Some metabolic events might perform as early drivers of epitranscriptional reprogramming; however, subsequent metabolic hits may govern the retention of stemness properties in the tumor mass. Interestingly, selective removal of mitochondria through autophagy can promote metabolic plasticity and alter metabolic states during differentiation and dedifferentiation. In this connection, novel metabostemness-specific drugs can be generated as potential cancer therapeutics to target the metaboloepigenetic circuitry to eliminate CSCs.
Graphical abstract






Similar content being viewed by others
References
Naik, P. P., Birbrair, A., & Bhutia, S. K. (2019). Mitophagy-driven metabolic switch reprograms stem cell fate. Cellular and Molecular Life Sciences, 76(1), 27–43.
Yang, M., Soga, T., & Pollard, P. J. (2013). Oncometabolites: linking altered metabolism with cancer. The Journal of Clinical Investigation, 123(9), 3652–3658.
Donohoe, D. R., & Bultman, S. J. (2012). Metaboloepigenetics: interrelationships between energy metabolism and epigenetic control of gene expression. Journal of Cellular Physiology, 227(9), 3169–3177.
Avitabile, D., Magenta, A., Lauri, A., Gambini, E., & Spaltro, G.Cristina Vinci, M. (2016). Metaboloepigenetics: the emerging network in stem cell homeostasis regulation. Current Stem Cell Research & Therapy, 11(4), 352–369.
Menendez, J. A., & Alarcón, T. (2014). Metabostemness: a new cancer hallmark. Frontiers in oncology, 4, 262.
Menendez, J. A., Corominas-Faja, B., Cuyàs, E., & Alarcón, T. (2014). Metabostemness: Metaboloepigenetic reprogramming of cancer stem-cell functions. Oncoscience, 1(12), 803.
Menendez, J. A. (2015). The metaboloepigenetic dimension of cancer stem cells: evaluating the market potential for new metabostemness-targeting oncology drugs. Current Pharmaceutical Design, 21(25), 3644–3653.
Menendez, J.A., & Joven, J. (2014). Energy metabolism and metabolic sensors in stem cells: the metabostem crossroads of aging and cancer. Advances in Experiemntal Medicine and Biology, 824, 117–140.
Menendez, J. A., & Alarcón, T. (2016). Nuclear reprogramming of cancer stem cells: Corrupting the epigenetic code of cell identity with oncometabolites. Molecular & cellular oncology, 3(6), e1160854.
Naik, P. P., Mukhopadhyay, S., Panda, P. K., Sinha, N., Das, C. K., Mishra, R., et al. (2018). Autophagy regulates cisplatin-induced stemness and chemoresistance via the upregulation of CD 44, ABCB 1 and ADAM 17 in oral squamous cell carcinoma. Cell proliferation, 51(1), e12411.
Naik, P. P., Mukhopadhyay, S., Praharaj, P. P., Bhol, C. S., Panigrahi, D. P., Mahapatra, K. K., et al. (2020). Secretory clusterin promotes oral cancer cell survival via inhibiting apoptosis by activation of autophagy in AMPK/mTOR/ULK1 dependent pathway. Life Sciences, 264.
Naik, P. P. (2020). Mitophagy and Reverse Warburg Effect: Metabolic Compartmentalization of Tumor Microenvironment. In Autophagy in tumor and tumor microenvironment, 117–140, Springer.
Naik, P. P., Panda, P. K., & Bhutia, S. K. (2017). Oral cancer stem cells microenvironment. In Stem cell microenvironments and beyond, 207–233, Springer.
Naik, P. P., Das, D. N., Panda, P. K., Mukhopadhyay, S., Sinha, N., Praharaj, P. P., et al. (2016). Implications of cancer stem cells in developing therapeutic resistance in oral cancer. Oral Oncology, 62, 122–135.
Naik, P. P., Praharaj, P. P., Bhol, C. S., Panigrahi, D. P., Mahapatra, K. K., Patra, S., et al. (2019). Mitochondrial Heterogeneity in Stem Cells. Advnces in Experiemntal Medecine and Biology, 1123, 179–194.
Menendez, J. A. (2015). Metabolic control of cancer cell stemness: Lessons from iPS cells. Cell Cycle, 14(24), 3801–3811.
Ciavardelli, D., Rossi, C., Barcaroli, D., Volpe, S., Consalvo, A., Zucchelli, M., et al. (2014). Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death and Diseases, 5(7), e1336.
Menendez, J. A., Joven, J., Cufí, S., Corominas-Faja, B., Oliveras-Ferraros, C., Cuyàs, E., et al. (2013). The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells. Cell Cycle, 12(8), 1166–1179.
Jang, Y. Y., & Sharkis, S. J. (2007). A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood, 110(8), 3056–3063.
Bowie, M. B., McKnight, K. D., Kent, D. G., McCaffrey, L., Hoodless, P. A., & Eaves, C. J. (2006). Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. Journal of Clinical Investigation, 116(10), 2808–2816.
Chen, C., Liu, Y., Liu, R., Ikenoue, T., Guan, K. L., Liu, Y., et al. (2008). TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. Journal of Experimental Medicine, 205(10), 2397–2408.
Kobayashi, C. I., & Suda, T. (2012). Regulation of reactive oxygen species in stem cells and cancer stem cells. Journal of Cellular Physiology, 227(2), 421–430.
Emmink, B. L., Verheem, A., Van Houdt, W. J., Steller, E. J., Govaert, K. M., Pham, T. V., et al. (2013). The secretome of colon cancer stem cells contains drug-metabolizing enzymes. Journal of Proteomics, 91, 84–96.
Liao, J., Qian, F., Tchabo, N., Mhawech-Fauceglia, P., Beck, A., Qian, Z., et al. (2014). Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One, 9(1), e84941.
Palorini, R., Votta, G., Balestrieri, C., Monestiroli, A., Olivieri, S., Vento, R., et al. (2014). Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. Journal of Cellular Biochemistry, 115(2), 368–379.
Zhou, Y., Zhou, Y., Shingu, T., Feng, L., Chen, Z., Ogasawara, M., et al. (2011). Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. Jornal of Biological Chemistry, 286(37), 32843–32853.
Martinez-Outschoorn, U. E., Peiris-Pagés, M., Pestell, R. G., Sotgia, F., & Lisanti, M. P. (2017). Cancer metabolism: a therapeutic perspective. Nature Reviews Clinical Oncology, 14(2), 113.
Sancho, P., Barneda, D., & Heeschen, C. (2016). Hallmarks of cancer stem cell metabolism. British Journal of Cancer, 114(12), 1305–1312.
Peiris-Pagès, M., Martinez-Outschoorn, U. E., Pestell, R. G., Sotgia, F., & Lisanti, M. P. (2016). Cancer stem cell metabolism. Breast Cancer Research, 18(1), 55.
Liu, P. P., Liao, J., Tang, Z. J., Wu, W. J., Yang, J., Zeng, Z. L., et al. (2014). Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death and Differentiation, 21(1), 124–135.
Tamada, M., Nagano, O., Tateyama, S., Ohmura, M., Yae, T., Ishimoto, T., et al. (2012). Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Research, 72(6), 1438–1448.
Zhao, H., Duan, Q., Zhang, Z., Li, H., Wu, H., Shen, Q., et al. (2017). Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. Journal of Cellular and Molecular Medicine, 21(9), 2055–2067.
Sowa, T., Menju, T., Chen-Yoshikawa, T. F., Takahashi, K., Nishikawa, S., Nakanishi, T., et al. (2017). Hypoxia-inducible factor 1 promotes chemoresistance of lung cancer by inducing carbonic anhydrase IX expression. Cancer Medicine, 6(1), 288–297.
Dong, C., Yuan, T., Wu, Y., Wang, Y., Fan, T. W., Miriyala, S., et al. (2013). Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell, 23(3), 316–331.
Shen, Y. A., Wang, C. Y., Hsieh, Y. T., Chen, Y. J., & Wei, Y. H. (2015). Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle, 14(1), 86–98.
Goidts, V., Bageritz, J., Puccio, L., Nakata, S., Zapatka, M., Barbus, S., et al. (2012). RNAi screening in glioma stem-like cells identifies PFKFB4 as a key molecule important for cancer cell survival. Oncogene, 31(27), 3235–3243.
Xu, Q., Tu, J., Dou, C., Zhang, J., Yang, L., Liu, X., et al. (2017). HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Molecular Cancer, 16(1), 178.
Liu, X., Chen, S., Tu, J., Cai, W., & Xu, Q. (2016). HSP90 inhibits apoptosis and promotes growth by regulating HIF-1α abundance in hepatocellular carcinoma. International Journal of Molecular Medicine, 37(3), 825–835.
Canal, F., & Perret, C. (2012). PKM2: a new player in the β-catenin game. Future Oncology, 8(4), 395–398.
Lee, J., Kim, H. K., Han, Y. M., & Kim, J. (2008). Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. The International Journal of Biochemistry & Cell Biology, 40(5), 1043–1054.
Yang, W., Xia, Y., Ji, H., Zheng, Y., Liang, J., Huang, W., et al. (2017). Corrigendum: Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature, 550(7674), 142.
Denko, N. C. (2008). Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Reviews Cancer, 8(9), 705–713.
Meijer, T. W., Kaanders, J. H., Span, P. N., & Bussink, J. (2012). Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clinical Cancer Research, 18(20), 5585–5594.
Singh, D., Arora, R., Kaur, P., Singh, B., Mannan, R., & Arora, S. (2017). Overexpression of hypoxia-inducible factor and metabolic pathways: possible targets of cancer. Cell and Bioscience, 7, 62.
Kim, N. H., Cha, Y. H., Lee, J., Lee, S. H., Yang, J. H., Yun, J. S., et al. (2017). Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. Nature Communication, 8, 14374.
Lee, S. Y., Jeong, E. K., Ju, M. K., Jeon, H. M., Kim, M. Y., Kim, C. H., et al. (2017). Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Molecular Cancer, 16(1), 10.
Lee, S. Y., Jeon, H. M., Ju, M. K., Jeong, E. K., Kim, C. H., Yoo, M. A., et al. (2015). Dlx-2 is implicated in TGF-β- and Wnt-induced epithelial-mesenchymal, glycolytic switch, and mitochondrial repression by Snail activation. International Journal of Oncology, 46(4), 1768–1780.
Lee, S. Y., Jeon, H. M., Ju, M. K., Jeong, E. K., Kim, C. H., Park, H. G., et al. (2016). Dlx-2 and glutaminase upregulate epithelial-mesenchymal transition and glycolytic switch. Oncotarget, 7(7), 7925–7939.
Lu, J., Tan, M., & Cai, Q. (2015). The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Letters, 356, 156–164.
Lee, S. Y., Jeon, H. M., Ju, M. K., Kim, C. H., Yoon, G., Han, S. I., et al. (2012). Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Research, 72(14), 3607–3617.
Thompson, C. B. (2014). Wnt meets Warburg: another piece in the puzzle? The EMBO Journal, 33(13), 1420–1422.
Pate, K. T., Stringari, C., Sprowl-Tanio, S., Wang, K., TeSlaa, T., Hoverter, N. P., et al. (2014). Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. The EMBO Journal, 33(13), 1454–1473.
Sherwood, V. (2015). WNT signaling: an emerging mediator of cancer cell metabolism? Molecular Cell Biology, 35(1), 2–10.
Kondaveeti, Y., Guttilla, R., White, I. K., & B. A. (2015). Epithelial-mesenchymal transition induces similar metabolic alterations in two independent breast cancer cell lines. Cancer Letters, 364(1), 44–58.
Luo, W., Hu, H., Chang, R., Zhong, J., Knabel, M., O'Meally, R., et al. (2011). Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell, 145(5), 732–744.
Luo, W., & Semenza, G. L. (2011). Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget, 2(7), 551–556.
Demaria, M., Misale, S., Giorgi, C., Miano, V., Camporeale, A., Campisi, J., et al. (2012). STAT3 can serve as a hit in the process of malignant transformation of primary cells. Cell Death and Differentiation, 19(8), 1390–1397.
Hamabe, A., Konno, M., Tanuma, N., Shima, H., Tsunekuni, K., Kawamoto, K., et al. (2014). Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition. Proceedings of the National Academy of Sciences of the United States of America, 111(43), 15526–15531.
Li, Q., Li, Y., Xu, J., Wang, S., Xu, Y., Li, X., et al. (2017). Aldolase B Overexpression is Associated with Poor Prognosis and Promotes Tumor Progression by Epithelial-Mesenchymal Transition in Colorectal Adenocarcinoma. Cellular Physiology and Biochemistry, 42(1), 397–406.
Ye, F., Chen, Y., Xia, L., Lian, J., & Yang, S. (2018). Aldolase A overexpression is associated with poor prognosis and promotes tumor progression by the epithelial-mesenchymal transition in colon cancer. Biochemical and Biophysical Research Communications, 497(2), 639–645.
Jiang, F., Ma, S., Xue, Y., Hou, J., & Zhang, Y. (2016). LDH-A promotes malignant progression via activation of epithelial-to-mesenchymal transition and conferring stemness in muscle-invasive bladder cancer. Biochemical and Biophysical Research Communications, 469(4), 985–992.
Janiszewska, M., Suvà, M. L., Riggi, N., Houtkooper, R. H., Auwerx, J., Clément-Schatlo, V., et al. (2012). Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes & Development, 26(17), 1926–1944.
Lagadinou, E. D., Sach, A., Callahan, K., Rossi, R. M., Neering, S. J., Minhajuddin, M., et al. (2013). BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell, 12(3), 329–341.
Sancho, P., Burgos-Ramos, E., Tavera, A., Bou Kheir, T., Jagust, P., Schoenhals, M., et al. (2015). MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metabolism, 22(4), 590–605.
Viale, A., Pettazzoni, P., Lyssiotis, C. A., Ying, H., Sánchez, N., Marchesini, M., et al. (2014). Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature, 514(7524), 628–632.
Pastò, A., Bellio, C., Pilotto, G., Ciminale, V., Silic-Benussi, M., Guzzo, G., et al. (2014). Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget, 5(12), 4305–4319.
Sato, M., Kawana, K., Adachi, K., Fujimoto, A., Yoshida, M., Nakamura, H., et al. (2016). Spheroid cancer stem cells display reprogrammed metabolism and obtain energy by actively running the tricarboxylic acid (TCA) cycle. Oncotarget, 7(22), 33297–33305.
Gao, C., Shen, Y., Jin, F., Miao, Y., & Qiu, X. (2016). Cancer Stem Cells in Small Cell Lung Cancer Cell Line H446: Higher Dependency on Oxidative Phosphorylation and Mitochondrial Substrate-Level Phosphorylation than Non-Stem Cancer Cells. PLoS One, 11(5), e0154576.
Lee, K. M., Giltnane, J. M., Balko, J. M., Schwarz, L. J., Guerrero-Zotano, A. L., Hutchinson, K. E., et al. (2017). MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metabolism, 26(4), 633–647.e637.
LeBleu, V. S., O'Connell, J. T., Gonzalez Herrera, K. N., Wikman, H., Pantel, K., Haigis, M. C., et al. (2014). PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nature Cell Biology, 16(10), 992–1003.
Jiang, W. G., Douglas-Jones, A., & Mansel, R. E. (2003). Expression of peroxisome-proliferator activated receptor-gamma (PPARgamma) and the PPARgamma co-activator, PGC-1, in human breast cancer correlates with clinical outcomes. International Journal of Cancer, 106(5), 752–757.
De Luca, A., Fiorillo, M., Peiris-Pagès, M., Ozsvari, B., Smith, D. L., Sanchez-Alvarez, R., et al. (2015). Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget, 6(17), 14777–14795.
Lamb, R., Harrison, H., Hulit, J., Smith, D. L., Lisanti, M. P., & Sotgia, F. (2014). Mitochondria as new therapeutic targets for eradicating cancer stem cells: Quantitative proteomics and functional validation via MCT1/2 inhibition. Oncotarget, 5(22), 11029–11037.
Vlashi, E., Lagadec, C., Vergnes, L., Reue, K., Frohnen, P., Chan, M., et al. (2014). Metabolic differences in breast cancer stem cells and differentiated progeny. Breast Cancer Research and Treatment, 146(3), 525–534.
Farnie, G., Sotgia, F., & Lisanti, M. P. (2015). High mitochondrial mass identifies a sub-population of stem-like cancer cells that are chemo-resistant. Oncotarget, 6(31), 30472–30486.
Vazquez, F., Lim, J. H., Chim, H., Bhalla, K., Girnun, G., Pierce, K., et al. (2013). PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell, 23(3), 287–301.
Chen, C. L., Uthaya Kumar, D. B., Punj, V., Xu, J., Sher, L., Tahara, S. M., et al. (2016). NANOG Metabolically Reprograms Tumor-Initiating Stem-like Cells through Tumorigenic Changes in Oxidative Phosphorylation and Fatty Acid Metabolism. Cell Metabolism, 23(1), 206–219.
El Hout, M., Cosialls, E., Mehrpour, M., & Hamaï, A. (2020). Crosstalk between autophagy and metabolic regulation of cancer stem cells. Molecular Cancer, 19(1), 27.
Bonuccelli, G., Peiris-Pages, M., Ozsvari, B., Martinez-Outschoorn, U. E., Sotgia, F., & Lisanti, M. P. (2017). Targeting cancer stem cell propagation with palbociclib, a CDK4/6 inhibitor: Telomerase drives tumor cell heterogeneity. Oncotarget, 8(6), 9868–9884.
Snyder, V., Reed-Newman, T. C., Arnold, L., Thomas, S. M., & Anant, S. (2018). Cancer Stem Cell Metabolism and Potential Therapeutic Targets. Frontiers in Oncology, 8, 203.
Yajima, T., Ochiai, H., Uchiyama, T., Takano, N., Shibahara, T., & Azuma, T. (2009). Resistance to cytotoxic chemotherapy-induced apoptosis in side population cells of human oral squamous cell carcinoma cell line Ho-1-N-1. International Journal of Oncology, 35(2), 273–280.
Zhang, G., Frederick, D. T., Wu, L., Wei, Z., Krepler, C., Srinivasan, S., et al. (2016). Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. Journal of Clinical Investigation, 126(5), 1834–1856.
Corominas-Faja, B., Cuyàs, E., Gumuzio, J., Bosch-Barrera, J., Leis, O., Martin, Á., & G., et al. (2014). Chemical inhibition of acetyl-CoA carboxylase suppresses self-renewal growth of cancer stem cells. Oncotarget, 5(18), 8306–8316.
Corominas-Faja, B., Vellon, L., Cuyàs, E., Buxó, M., Martin-Castillo, B., Serra, D., et al. (2017). Clinical and therapeutic relevance of the metabolic oncogene fatty acid synthase in HER2+ breast cancer. Histology & Histopathology, 32(7), 687–698.
González-Bártulos, M., Aceves-Luquero, C., Qualai, J., Cussó, O., Martínez, M. A., Fernández de Mattos, S., et al. (2015). Pro-Oxidant Activity of Amine-Pyridine-Based Iron Complexes Efficiently Kills Cancer and Cancer Stem-Like Cells. PLoS One, 10(9), e0137800.
Yi, M., Li, J., Chen, S., Cai, J., Ban, Y., Peng, Q., et al. (2018). Emerging role of lipid metabolism alterations in Cancer stem cells. Journal of Experimental & Clinical Cancer Research, 37(1), 118.
Pandey, P. R., Xing, F., Sharma, S., Watabe, M., Pai, S. K., Iiizumi-Gairani, M., et al. (2013). Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene, 32(42), 5111–5122.
Wang, T., Fahrmann, J. F., Lee, H., Li, Y. J., Tripathi, S. C., Yue, C., et al. (2018). JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metabolism, 27(1), 136–150.
Gonzalez-Guerrico, A. M., Espinoza, I., Schroeder, B., Park, C. H., Kvp, C. M., Khurana, A., et al. (2016). Suppression of endogenous lipogenesis induces reversion of the malignant phenotype and normalized differentiation in breast cancer. Oncotarget, 7(44), 71151–71168.
Yang, L., Zhang, F., Wang, X., Tsai, Y., Chuang, K. H., Keng, P. C., et al. (2016). A FASN-TGF-β1-FASN regulatory loop contributes to high EMT/metastatic potential of cisplatin-resistant non-small cell lung cancer. Oncotarget, 7(34), 55543–55554.
Gao, P., Tchernyshyov, I., Chang, T. C., Lee, Y. S., Kita, K., Ochi, T., et al. (2009). c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature, 458(7239), 762–765.
Peixoto, J., & Lima, J. (2018). Metabolic traits of cancer stem cells. Disease Models & Mechanisms, 11(8), 033464.
Cuyàs, E., Verdura, S., Fernández-Arroyo, S., Bosch-Barrera, J., Martin-Castillo, B., Joven, J., et al. (2017). Metabolomic mapping of cancer stem cells for reducing and exploiting tumor heterogeneity. Oncotarget, 8(59), 99223–99236.
Goding, C. R., Pei, D., & Lu, X. (2014). Cancer: pathological nuclear reprogramming? Nature Reviews Cancer, 14(8), 568–573.
Saha, S. K., Parachoniak, C. A., & Bardeesy, N. (2014). IDH mutations in liver cell plasticity and biliary cancer. Cell Cycle., 13(20), 3176–3182.
Huang, S. (2009). Reprogramming cell fates: reconciling rarity with robustness. Bioessays, 31(5), 546–560.
Huang, S., Ernberg, I., & Kauffman, S. (2009). Cancer attractors: a systems view of tumors from a gene network dynamics and developmental perspective. Seminars in Cell and Developmental Biology, 20(7), 869–876.
Hanna, J. H., Saha, K., & Jaenisch, R. (2010). Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell, 143(4), 508–525.
Huang, S. (2012). The molecular and mathematical basis of Waddington's epigenetic landscape: a framework for post-Darwinian biology? Bioessays, 34(2), 149–157.
Lu, C., & Thompson, C. B. (2012). Metabolic regulation of epigenetics. Cell Metabolism, 16(1), 9–17.
Menendez, J. A., Alarcón, T., & Joven, J. (2014). Gerometabolites: the pseudohypoxic aging side of cancer oncometabolites. Cell Cycle, 13(5), 699–709.
Nowicki, S., & Gottlieb, E. (2015). Oncometabolites: tailoring our genes. The FEBS Journal, 282(15), 2796–2805.
Nam, H., Campodonico, M., Bordbar, A., Hyduke, D. R., Kim, S., Zielinski, D. C., et al. (2014). A systems approach to predict oncometabolites via context-specific genome-scale metabolic networks. PLoS Computational Biology, 10(9), e1003837.
Corominas-Faja, B., Cufí, S., Oliveras-Ferraros, C., Cuyàs, E., López-Bonet, E., Lupu, R., et al. (2013). Nuclear reprogramming of luminal-like breast cancer cells generates Sox2-overexpressing cancer stem-like cellular states harboring transcriptional activation of the mTOR pathway. Cell Cycle, 12(18), 3109–3124.
Terunuma, A., Putluri, N., Mishra, P., Mathé, E. A., Dorsey, T. H., Yi, M., et al. (2014). MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. Journal of Clinincal Investigation, 124(1), 398–412.
Lee, S. Y., Ju, M. K., Jeon, H. M., Lee, Y. J., Kim, C. H., Park, H. G., et al. (2018). Oncogenic Metabolism Acts as a Prerequisite Step for Induction of Cancer Metastasis and Cancer Stem Cell Phenotype. Oxidative Medicine and Cellular Longevity, 23, 1027453.
Røsland, G. V., Dyrstad, S. E., Tusubira, D., Helwa, R., Tan, T. Z., Lotsberg, M. L., et al. (2019). Epithelial to mesenchymal transition (EMT) is associated with attenuation of succinate dehydrogenase (SDH) in breast cancer through reduced expression of SDHC. Cancer Metabolism, 7, 6.
Ahmad, A., Aboukameel, A., Kong, D., Wang, Z., Sethi, S., Chen, W., et al. (2011). Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Research, 71(9), 3400–3409.
Goel, A., Mathupala, S. P., & Pedersen, P. L. (2003). Glucose metabolism in cancer. Evidence that demethylation events play a role in activating type II hexokinase gene expression. Journal of Biological Chemistry, 278(17), 15333–15340.
Liu, X., Wang, X., Zhang, J., Lam, E. K., Shin, V. Y., Cheng, A. S., et al. (2010). Warburg effect revisited: an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene, 29(3), 442–450.
Wolf, A., Agnihotri, S., Munoz, D., & Guha, A. (2011). Developmental profile and regulation of the glycolytic enzyme hexokinase 2 in normal brain and glioblastoma multiforme. Neurobiology of Disease, 44(1), 84–91.
Chen, M., Zhang, J., Li, N., Qian, Z., Zhu, M., Li, Q., et al. (2011). Promoter hypermethylation mediated downregulation of FBP1 in human hepatocellular carcinoma and colon cancer. PLoS One, 6(10), e25564.
Lopez-Serra, P., Marcilla, M., Villanueva, A., Ramos-Fernandez, A., Palau, A., Leal, L., et al. (2014). A DERL3-associated defect in the degradation of SLC2A1 mediates the Warburg effect. Nature Communication, 5, 3608.
Hong, H., Takahashi, K., Ichisaka, T., Aoi, T., Kanagawa, O., Nakagawa, M., et al. (2009). Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature, 460(7259), 1132–1135.
Kawamura, T., Suzuki, J., Wang, Y. V., Menendez, S., Morera, L. B., Raya, A., et al. (2009). Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature, 460(7259), 1140–1144.
Li, H., Collado, M., Villasante, A., Strati, K., Ortega, S., Cañamero, M., et al. (2009). The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature, 460(7259), 1136–1139.
Marión, R. M., Strati, K., Li, H., Murga, M., Blanco, R., Ortega, S., et al. (2009). A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature, 460(7259), 1149–1153.
Utikal, J., Polo, J. M., Stadtfeld, M., Maherali, N., Kulalert, W., Walsh, R. M., et al. (2009). Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature, 460(7259), 1145–1148.
Sarig, R., Rivlin, N., Brosh, R., Bornstein, C., Kamer, I., Ezra, O., et al. (2010). Mutant p53 facilitates somatic cell reprogramming and augments the malignant potential of reprogrammed cells. Journal of Experimental Medicine, 207(10), 2127–2140.
Tapia, N., & Schöler, H. R. (2010). p53 connects tumorigenesis and reprogramming to pluripotency. Journal of Experimental Medicine, 207(10), 2045–2048.
Bensaad, K., Tsuruta, A., Selak, M. A., Vidal, M. N., Nakano, K., Bartrons, R., et al. (2006). TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell, 126(1), 107–120.
Kawauchi, K., Araki, K., Tobiume, K., & Tanaka, N. (2008). p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nature Cell Biology, 10(5), 611–618.
Maddocks, O. D., & Vousden, K. H. (2011). Metabolic regulation by p53. Berl Journal of Molecular Medicine, 89(3), 237–245.
Sen, N., Satija, Y. K., & Das, S. (2011). PGC-1α, a key modulator of p53, promotes cell survival upon metabolic stress. Molecular Cell, 44(4), 621–634.
Sen, N., Satija, Y. K., & Das, S. (2012). p53 and metabolism: old player in a new game. Transcription, 3(3), 119–123.
Berkers, C. R., Maddocks, O. D., Cheung, E. C., Mor, I., & Vousden, K. H. (2013). Metabolic regulation by p53 family members. Cell Metabolism, 18(5), 617–633.
Hitchler, M. J., & Domann, F. E. (2009). Metabolic defects provide a spark for the epigenetic switch in cancer. Free Radical Biology and Medicine, 47(2), 115–127.
Sauve, A. A., Wolberger, C., Schramm, V. L., & Boeke, J. D. (2006). The biochemistry of sirtuins. Annual Review of Biochemistry, 75, 435–465.
Alemasova, E. E., & Lavrik, O. I. (2019). Poly(ADP-ribosyl)ation by PARP1: reaction mechanism and regulatory proteins. Nucleic Acids Research, 47(8), 3811–3827.
Belenky, P., Bogan, K. L., & Brenner, C. (2007). NAD+ metabolism in health and disease. Trends in Biochemical Sciences, 32(1), 12–19.
Quénet, D., El Ramy, R., Schreiber, V., & Dantzer, F. (2009). The role of poly(ADP-ribosyl)ation in epigenetic events. The International Journal of Biochemistry & Cell Biology, 41(1), 60–65.
Li, X., & Kazgan, N. (2011). Mammalian sirtuins and energy metabolism. International Journal of Biological Sciences, 7(5), 575–587.
Teperino, R., Schoonjans, K., & Auwerx, J. (2010). Histone methyl transferases and demethylases; can they link metabolism and transcription? Cell Metabolism, 12(4), 321–327.
Huang, J., Sengupta, R., Espejo, A. B., Lee, M. G., Dorsey, J. A., Richter, M., et al. (2007). p53 is regulated by the lysine demethylase LSD1. Nature, 449(7158), 105–108.
Kim, S. C., Sprung, R., Chen, Y., Xu, Y., Ball, H., Pei, J., et al. (2006). Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Molecular Cell, 23(4), 607–618.
Wellen, K. E., Hatzivassiliou, G., Sachdeva, U. M., Bui, T. V., Cross, J. R., & Thompson, C. B. (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science, 324(5930), 1076–1080.
Zhao, S., Xu, W., Jiang, W., Yu, W., Lin, Y., Zhang, T., et al. (2010). Regulation of cellular metabolism by protein lysine acetylation. Science, 327(5968), 1000–1004.
Choudhary, C., Kumar, C., Gnad, F., Nielsen, M. L., Rehman, M., Walther, T. C., et al. (2009). Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science, 325(5942), 834–840.
Wallace, D. C., & Fan, W. (2010). Energetics, epigenetics, mitochondrial genetics. Mitochondrion, 10(1), 12–31.
Ward, P., S., Patel, J., Wise, D., R., Abdel-Wahab, O., Bennett, B.D., Coller, H., A., et al. (2010). The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell, 17(3), 225–34.
Xu, W., Yang, H., Liu, Y., Yang, Y., Wang, P., Kim, S. H., et al. (2011). Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell, 19(1), 17–30.
Chowdhury, R., Yeoh, K. K., Tian, Y. M., Hillringhaus, L., Bagg, E. A., Rose, N. R., et al. (2011). The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Report, 12(5), 463–469.
Frezza, C., Pollard, P. J., & Gottlieb, E. (2011). Inborn and acquired metabolic defects in cancer. Journal of Molecular Medicine, 89(3), 213–220.
Yang, M., Soga, T., Pollard, P. J., & Adam, J. (2012). The emerging role of fumarate as an oncometabolite. Frontiers in Oncology, 2, 85.
Cairns, R. A., & Mak, T. W. (2013). Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discovery, 3(7), 730–741.
Krell, D., Mulholland, P., Frampton, A. E., Krell, J., Stebbing, J., & Bardella, C. (2013). IDH mutations in tumorigenesis and their potential role as novel therapeutic targets. Future Oncology, 9(12), 1923–1935.
Sullivan, L. B., Martinez-Garcia, E., Nguyen, H., Mullen, A. R., Dufour, E., Sudarshan, S., et al. (2013). The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Molecular Cell, 51(2), 236–248.
Adam, J., Yang, M., Soga, T., & Pollard, P. J. (2014). Rare insights into cancer biology. Oncogene, 33(20), 2547–2556.
Borger, D. R., Goyal, L., Yau, T., Poon, R. T., Ancukiewicz, M., Deshpande, V., et al. (2014). Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Cliical Cancer Research, 20(7), 1884–1890.
Gaude, E., & Frezza, C. (2014). Defects in mitochondrial metabolism and cancer. Cancer Metabolism, 2, 10.
Saha, S. K., Parachoniak, C. A., Ghanta, K. S., Fitamant, J., Ross, K. N., Najem, M. S., et al. (2014). Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature, 513(7516), 110–114.
Menendez, J. A., Corominas-Faja, B., Cuyàs, E., García, M. G., Fernández-Arroyo, S., Fernández, A. F., et al. (2016). Oncometabolic Nuclear Reprogramming of Cancer Stemness. Stem Cell Reports, 6(3), 273–283.
Youle, R. J., & Narendra, D. P. (2011). Mechanisms of mitophagy. Nature Reviews Molecular Cell Biology, 12(1), 9–14.
Zhang, H., Bosch-Marce, M., Shimoda, L. A., Tan, Y. S., Baek, J. H., Wesley, J. B., et al. (2008). Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. Journal of Biological Chemistry, 283(16), 10892–10903.
Vazquez-Martin, A., Van den Haute, C., Cufí, S., Corominas-Faja, B., Cuyàs, E., Lopez-Bonet, E., et al. (2016). Mitophagy-driven mitochondrial rejuvenation regulates stem cell fate. Aging (Albany NY), 8(7), 1330.
Vazquez-Martin, A., Cufí, S., Corominas-Faja, B., Oliveras-Ferraros, C., Vellon, L., & Menendez, J. A. (2012). Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging (Albany NY), 4(6), 393.
Lee, Y. J., Jeong, S.-Y., Karbowski, M., Smith, C. L., & Youle, R. J. (2004). Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Molecular Biology of the Cell, 15(11), 5001–5011.
Folmes, C. D., Nelson, T. J., Martinez-Fernandez, A., Arrell, D. K., Lindor, J. Z., Dzeja, P. P., et al. (2011). Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metabolism, 14(2), 264–271.
Son, M. J., Kwon, Y., Son, M. Y., Seol, B., Choi, H. S., Ryu, S. W., et al. (2015). Mitofusins deficiency elicits mitochondrial metabolic reprogramming to pluripotency. Cell Death and Differiation, 22(12), 1957–1969.
Zhou, J., Li, G., Zheng, Y., Shen, H. M., Hu, X., Ming, Q. L., et al. (2015). A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy, 11(8), 1259–1279.
Chang, C. M., Lan, K. L., Huang, W. S., Lee, Y. J., Lee, T. W., Chang, C. H., et al. (2017). 188Re-Liposome Can Induce Mitochondrial Autophagy and Reverse Drug Resistance for Ovarian Cancer: From Bench Evidence to Preliminary Clinical Proof-of-Concept. International Journal of Molecular Sciences, 18(5), 903.
Chen, Y. Y., Wang, W. H., Che, L., Lan, Y., Zhang, L. Y., Zhan, D. L., et al. (2020). BNIP3L-Dependent Mitophagy Promotes HBx-Induced Cancer Stemness of Hepatocellular Carcinoma Cells via Glycolysis Metabolism Reprogramming. Cancers (Basel), 12(3), 655.
Alcalá, S., Sancho, P., Martinelli, P., Navarro, D., Pedrero, C., Martín-Hijano, L., et al. (2020). ISG15 and ISGylation is required for pancreatic cancer stem cell mitophagy and metabolic plasticity. Nature Communication, 11(1), 2682.
Wang, L., Zhang, T., Wang, L., Cai, Y., Zhong, X., He, X., et al. (2017). Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission. The EMBO Journal, 36(10), 1330–1347.
Picard, F., Kurtev, M., Chung, N., Topark-Ngarm, A., Senawong, T., Machado De Oliveira, R., et al. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature, 429(6993), 771–776.
Vaziri, H., Dessain, S. K., Ng Eaton, E., Imai, S. I., Frye, R. A., Pandita, T. K., et al. (2001). hSIR2 (SIRT1) functions as an NAD-dependent p53 deacetylase. Cell, 107(2), 149–159.
Brunet, A., Sweeney, L. B., Sturgill, J. F., Chua, K. F., Greer, P. L., Lin, Y., et al. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science, 303(5666), 2011–2015.
Aguilar, E., Marin de Mas, I., Zodda, E., Marin, S., Morrish, F., Selivanov, V., et al. (2016). Metabolic Reprogramming and Dependencies Associated with Epithelial Cancer Stem Cells Independent of the Epithelial-Mesenchymal Transition Program. Stem Cells, 34(5), 1163–1176.
Jang, S. Y., Kang, H. T., & Hwang, E. S. (2012). Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. Journal of Biological Chemistry, 287(23), 19304–19314.
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 324(5930), 1029–1033.
Shackelford, D. B., Vasquez, D. S., Corbeil, J., Wu, S., Leblanc, M., Wu, C. L., et al. (2009). mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proceedings of the National Academy of Sciences of the United States of America, 106(27), 11137–11142.
Chen, W. J., & Huang, R. S. (2018). Low-folate stress reprograms cancer stem cell-like potentials and bioenergetics metabolism through activation of mTOR signaling pathway to promote in vitro invasion and in vivo tumorigenicity of lung cancers. Journal of Nutritional Biochemistry, 53, 28–38.
Zgheib, R., Battaglia-Hsu, S. F., Hergalant, S., Quéré, M., Alberto, J. M., Chéry, C., et al. (2019). Folate can promote the methionine-dependent reprogramming of glioblastoma cells towards pluripotency. Cell Death and Diseases, 10(8), 596.
Acknowledgments
We apologize to the authors for any fundamental studies that were omitted due to space limitations. Research support was partly provided by the Department of Biotechnology [BT/PR23304/MED/30/1823/2017], Ministry of Science and Technology, Government of India.
Availability of data and material
Not Applicable
Funding
Research support was provided by the Department of Biotechnology [BT/PR23304/MED/30/1823/2017], Ministry of Science and Technology, Government of India.
Author information
Authors and Affiliations
Contributions
PPN, PPP, CSB and SKB conceived the review. PPN, SP, RP, PPP CSB and SKB wrote the review, centralized and integrated comments from co-authors, and prepared the figures and tables. SP, MN, DG, SKP and SKB corrected the manuscript and provided valuable input to obtain a consensus view.
Corresponding author
Ethics declarations
Competing interests
The authors disclose no conflict of interest.
Ethics approval and consent to participate
Not Applicable
Consent for publication
All Authors give consent to publish the Work
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naik, P.P., Panigrahi, S., Parida, R. et al. Metabostemness in cancer: Linking metaboloepigenetics and mitophagy in remodeling cancer stem cells . Stem Cell Rev and Rep 18, 198–213 (2022). https://doi.org/10.1007/s12015-021-10216-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10216-9