Abstract
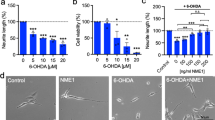
A greater understanding of the mechanisms that promote the survival and growth of dopaminergic neurons is essential for the advancement of cell replacement therapies for Parkinson’s disease (PD). Evidence supports a role for the mitogen-activated protein kinase p38 in the demise of dopaminergic neurons, while mitogen-activated protein kinase phosphatase-1 (MKP-1), which negatively regulates p38 activity, has not yet been investigated in this context. Here, we show that MKP-1 is expressed in dopaminergic neurons cultured from E14 rat ventral mesencephalon (VM). When dopaminergic neurons were transfected to overexpress MKP-1, they displayed a more complex morphology than their control counterparts in vitro. Specifically, MKP-1-transfection induced significant increases in neurite length and branching with a maximum increase observed in primary branches. We demonstrate that inhibition of dopaminergic neurite growth induced by treatment of rat VM neurons with the dopaminergic neurotoxin 6-hydroxydopamine (6-OHDA) in vitro is mediated by p38 and is concomitant with a significant and selective decrease in MKP-1 expression in these neurons. We further show that overexpression of MKP-1 in dopaminergic neurons contributes to neuroprotection against the effects of 6-OHDA. Collectively, we report that MKP-1 can promote the growth and elaboration of dopaminergic neuronal processes and can help protect them from the neurotoxic effects of 6-OHDA. Thus, we propose that strategies aimed at augmenting MKP-1 expression or activity may be beneficial in protecting dopaminergic neurons and may provide potential therapeutic approaches for PD.






Similar content being viewed by others
References
Bennett, B. L., Sasaki, D. T., Murray, B. W., O’Leary, E. C., Sakata, S. T., Xu, W., et al. (2001). SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proceedings of the National Academy of Sciences of the United States of America, 98(24), 13681–13686.
Blandini, F., Porter, R. H., & Greenamyre, J. T. (1996). Glutamate and Parkinson’s disease. Molecular Neurobiology, 12(1), 73–94.
Blum, D., Torch, S., Lambeng, N., Nissou, M., Benabid, A. L., Sadoul, R., et al. (2001). Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Progress in Neurobiology, 65(2), 135–172.
Camps, M., Nichols, A., & Arkinstall, S. (2000). Dual specificity phosphatases: A gene family for control of MAP kinase function. FASEB journal, 14(1), 6–16.
Chambers, J. W., Pachori, A., Howard, S., Iqbal, S., & Lograsso, P. V. (2013). Inhibition of JNK mitochondrial localization and signaling is protective against ischemia/reperfusion injury in rats. The Journal of biological chemistry, 288(6), 4000–4011.
Che, W., Manetsch, M., Quante, T., Rahman, M. M., Patel, B. S., Ge, Q., et al. (2012). Sphingosine 1-phosphate induces MKP-1 expression via p38 MAPK- and CREB-mediated pathways in airway smooth muscle cells. Biochimica et Biophysica Acta, 1823(10), 1658–1665.
Choi, W. S., Eom, D. S., Han, B. S., Kim, W. K., Han, B. H., Choi, E. J., et al. (2004). Phosphorylation of p38 MAPK induced by oxidative stress is linked to activation of both caspase-8- and -9-mediated apoptotic pathways in dopaminergic neurons. The Journal of biological chemistry, 279(19), 20451–20460.
Choi, B. H., Hur, E. M., Lee, J. H., Jun, D. J., & Kim, K. T. (2006). Protein kinase Cdelta-mediated proteasomal degradation of MAP kinase phosphatase-1 contributes to glutamate-induced neuronal cell death. Journal of Cell Science, 119(Pt 7), 1329–1340.
Chu, J. M., Chan, Y. S., Chen, L. W., & Yung, K. K. (2012). Neurokinin receptor 3 peptide exacerbates 6-hydroxydopamine-induced dopaminergic degeneration in rats through JNK pathway. Journal of Neurochemistry, 123(3), 417–427.
Coleman, M. (2005). Axon degeneration mechanisms: Commonality amid diversity. Nature Reviews Neuroscience, 6(11), 889–898.
Collins, L. M., Toulouse, A., Connor, T. J., & Nolan, Y. M. (2012). Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology, 62(7), 2154–2168.
Crampton, S. J., Collins, L. M., Toulouse, A., Nolan, Y. M., & O’Keeffe, G. W. (2012). Exposure of foetal neural progenitor cells to IL-1beta impairs their proliferation and alters their differentiation—a role for maternal inflammation? Journal of Neurochemistry, 120(6), 964–973.
Crocker, C. E., Khan, S., Cameron, M. D., Robertson, H. A., Robertson, G. S., & Lograsso, P. (2011). JNK inhibition protects dopamine neurons and provides behavioral improvement in a rat 6-hydroxydopamine model of Parkinson’s disease. ACS chemical neuroscience, 2(4), 207–212.
Crotty, S., Fitzgerald, P., Tuohy, E., Harris, D. M., Fisher, A., Mandel, A., et al. (2008). Neuroprotective effects of novel phosphatidylglycerol-based phospholipids in the 6-hydroxydopamine model of Parkinson’s disease. European Journal of Neuroscience, 27(2), 294–300.
Davis, S., Vanhoutte, P., Pages, C., Caboche, J., & Laroche, S. (2000). The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. The Journal of neuroscience, 20(12), 4563–4572.
Ding, Y. M., Jaumotte, J. D., Signore, A. P., & Zigmond, M. J. (2004). Effects of 6-hydroxydopamine on primary cultures of substantia nigra: Specific damage to dopamine neurons and the impact of glial cell line-derived neurotrophic factor. Journal of Neurochemistry, 89(3), 776–787.
Doddareddy, M., Rawling, T., & Ammit, A. J. (2012). Targeting mitogen-activated protein kinase phosphatase-1 (MKP-1): structure-based design of MKP-1 inhibitors and upregulators. Current Medicinal Chemistry, 19(2), 163–173.
Eljaschewitsch, E., Witting, A., Mawrin, C., Lee, T., Schmidt, P. M., Wolf, S., et al. (2006). The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron, 49(1), 67–79.
Farooq, A., & Zhou, M.-M. (2004). Structure and regulation of MAPK phosphatases. Cellular Signalling, 16(7), 769–779.
Franklin, C. C., & Kraft, A. S. (1997). Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. The Journal of biological chemistry, 272(27), 16917–16923.
Gallo, G. (2011). The cytoskeletal and signaling mechanisms of axon collateral branching. Developmental neurobiology, 71(3), 201–220.
Gass, P., Eckhardt, A., Schroder, H., Bravo, R., & Herdegen, T. (1996). Transient expression of the mitogen-activated protein kinase phosphatase MKP-1 (3CH134/ERP1) in the rat brain after limbic epilepsy. Brain Research. Molecular Brain Research, 41(1–2), 74–80.
Gutierrez, H., & Davies, A. (2007). A fast and accurate procedure for deriving the Sholl profile in quantitative studies of neuronal morphology. Journal of Neuroscience Methods, 163(1), 24–30.
Gutierrez, H., O'Keeffe, G. W., Gavalda, N., Gallagher, D., & Davies, A. M. (2008). Nuclear factor kappa B signaling either stimulates or inhibits neurite growth depending on the phosphorylation status of p65/RelA. Journal of Neuroscience, 28(33), 8246–8256.
Hutter, D., Chen, P., Barnes, J., & Liu, Y. (2000). Catalytic activation of mitogen-activated protein (MAP) kinase phosphatase-1 by binding to p38 MAP kinase: Critical role of the p38 C-terminal domain in its negative regulation. Biochemical journal, 352(Pt 1), 155.
Jeanneteau, F., & Deinhardt, K. (2011). Fine-tuning MAPK signaling in the brain: The role of MKP-1. Communicative and Integrative Biology, 4(3), 281–283.
Jeanneteau, F., Deinhardt, K., Miyoshi, G., Bennett, A. M., & Chao, M. V. (2010). The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nature Neuroscience, 13(11), 1373–1379.
Kim, H. J. (2011). Stem cell potential in Parkinson’s disease and molecular factors for the generation of dopamine neurons. Biochimica et Biophysica Acta, 1812(1), 1–11.
Lawan, A., Shi, H., Gatzke, F., & Bennett, A. M. (2012). Diversity and specificity of the mitogen-activated protein kinase phosphatase-1 functions. Cellular and Molecular Life Sciences, 70(2), 223–237.
Long-Smith, C. M., Collins, L., Toulouse, A., Sullivan, A. M., & Nolan, Y. M. (2010). Interleukin-1β contributes to dopaminergic neuronal death induced by lipopolysaccharide-stimulated rat glia in vitro. Journal of Neuroimmunology, 226(1–2), 20–26.
Mandel, S., Grunblatt, E., Riederer, P., Gerlach, M., Levites, Y., & Youdim, M. B. (2003). Neuroprotective strategies in Parkinson’s disease: An update on progress. CNS drugs, 17(10), 729–762.
Manetsch, M., Che, W., Seidel, P., Chen, Y., & Ammit, A. J. (2012). MKP-1: A negative feedback effector that represses MAPK-mediated pro-inflammatory signaling pathways and cytokine secretion in human airway smooth muscle cells. Cellular Signalling, 24(4), 907–913.
McKelvey, L., Gutierrez, H., Nocentini, G., Crampton, S. J., Davies, A. M., Riccardi, C. R., et al. (2012). The intracellular portion of GITR enhances NGF-promoted neurite growth through an inverse modulation of Erk and NF-kappaB signalling. Biology open, 1(10), 1016–1023.
Michel, P. P., & Hefti, F. (1990). Toxicity of 6-hydroxydopamine and dopamine for dopaminergic neurons in culture. Journal of Neuroscience Research, 26(4), 428–435.
Mishra, O. P., & Delivoria-Papadopoulos, M. (2004). Effect of hypoxia on the expression and activity of mitogen-activated protein (MAP) kinase-phosphatase-1 (MKP-1) and MKP-3 in neuronal nuclei of newborn piglets: The role of nitric oxide. Neuroscience, 129(3), 665–673.
Nolan, A. M., Nolan, Y. M., & O’Keeffe, G. W. (2011). IL-1beta inhibits axonal growth of developing sympathetic neurons. Molecular and cellular neurosciences, 48(2), 142–150.
Nolan, Y., Vereker, E., Lynch, A. M., & Lynch, M. A. (2003). Evidence that lipopolysaccharide-induced cell death is mediated by accumulation of reactive oxygen species and activation of p38 in rat cortex and hippocampus. Experimental Neurology, 184(2), 794–804.
O’Keeffe, G. W., Dockery, P., & Sullivan, A. M. (2004). Effects of growth/differentiation factor 5 on the survival and morphology of embryonic rat midbrain dopaminergic neurones. Journal of Neurocytology, 33(5), 479–488.
O’Keeffe, G. W., Gutierrez, H., Pandolfi, P. P., Riccardi, C., & Davies, A. M. (2008). NGF-promoted axon growth and target innervation requires GITRL-GITR signaling. Nature Neuroscience, 11(2), 135–142.
Owens, D. M., & Keyse, S. M. (2007). Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene, 26(22), 3203–3213.
Peinado-Ramon, P., Wallen, A., & Hallbook, F. (1998). MAP kinase phosphatase-1 mRNA is expressed in embryonic sympathetic neurons and is upregulated after NGF stimulation. Brain Research. Molecular Brain Research, 56(1–2), 256–267.
Pratt, P. F., Bokemeyer, D., Foschi, M., Sorokin, A., & Dunn, M. J. (2003). Alterations in subcellular localization of p38 MAPK potentiates endothelin-stimulated COX-2 expression in glomerular mesangial cells. The Journal of biological chemistry, 278(51), 51928–51936.
Raff, M. C., Whitmore, A. V., & Finn, J. T. (2002). Axonal self-destruction and neurodegeneration. Science, 296(5569), 868–871.
Rajadhyaksha, A., Husson, I., Satpute, S. S., Küppenbender, K. D., Ren, J. Q., Guerriero, R. M., et al. (2004). L-Type Ca2 + channels mediate adaptation of extracellular signal-regulated kinase 1/2 phosphorylation in the ventral tegmental area after chronic amphetamine treatment. The Journal of neuroscience, 24(34), 7464–7476.
Raman, M., Chen, W., & Cobb, M. H. (2007). Differential regulation and properties of MAPKs. Oncogene, 26(22), 3100–3112.
Rayport, S., Sulzer, D., Shi, W. X., Sawasdikosol, S., Monaco, J., Batson, D., et al. (1992). Identified postnatal mesolimbic dopamine neurons in culture: Morphology and electrophysiology. The Journal of neuroscience, 12(11), 4264–4280.
Ries, V., Silva, R. M., Oo, T. F., Cheng, H. C., Rzhetskaya, M., Kholodilov, N., et al. (2008). JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. Journal of Neurochemistry, 107(6), 1578–1588.
Slack, D. N., Seternes, O. M., Gabrielsen, M., & Keyse, S. M. (2001). Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. The Journal of biological chemistry, 276(19), 16491–16500.
Staples, C. J., Owens, D. M., Maier, J. V., Cato, A. C., & Keyse, S. M. (2010). Cross-talk between the p38alpha and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to UV radiation. The Journal of biological chemistry, 285(34), 25928–25940.
Takaki, M., Ujike, H., Kodama, M., Takehisa, Y., Nakata, K., & Kuroda, S. (2001). Two kinds of mitogen-activated protein kinase phosphatases, MKP-1 and MKP-3, are differentially activated by acute and chronic methamphetamine treatment in the rat brain. Journal of Neurochemistry, 79(3), 679–688.
Toulouse, A., & Sullivan, A. M. (2008). Progress in Parkinson’s disease-where do we stand? Progress in Neurobiology, 85(4), 376–392.
Valjent, E., Caboche, J., & Vanhoutte, P. (2001). Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: A molecular substrate for learning and memory? Molecular Neurobiology, 23(2–3), 83–99.
Walsh, S., Finn, D. P., & Dowd, E. (2011). Time-course of nigrostriatal neurodegeneration and neuroinflammation in the 6-hydroxydopamine-induced axonal and terminal lesion models of Parkinson’s disease in the rat. Neuroscience, 175, 251–261.
Wancket, L. M., Frazier, W. J., & Liu, Y. (2012). Mitogen-activated protein kinase phosphatase (MKP)-1 in immunology, physiology, and disease. Life Sciences, 90(7–8), 237–248.
Winter, C., Schenkel, J., Zimmermann, M., & Herdegen, T. (1998). MAP kinase phosphatase 1 is expressed and enhanced by FK506 in surviving mamillary, but not degenerating nigral neurons following axotomy. Brain Research, 801(1–2), 198–205.
Wu, J. J., Zhang, L., & Bennett, A. M. (2005). The noncatalytic amino terminus of mitogen-activated protein kinase phosphatase 1 directs nuclear targeting and serum response element transcriptional regulation. Molecular and Cellular Biology, 25(11), 4792–4803.
Acknowledgments
The authors would like to thank Ms Aisling Gavin for kindly providing us with tissue. This work was supported by the College of Medicine and Health, University College Cork, Science Foundation Ireland Grant No. SFI/RFP/NSC1298 (YN), Science Foundation Ireland Grant No. 10/RFP/NES2786 (GO’K), and the Irish Research Council for Science Engineering and Technology.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Collins, L.M., O’Keeffe, G.W., Long-Smith, C.M. et al. Mitogen-Activated Protein Kinase Phosphatase (MKP)-1 as a Neuroprotective Agent: Promotion of the Morphological Development of Midbrain Dopaminergic Neurons. Neuromol Med 15, 435–446 (2013). https://doi.org/10.1007/s12017-013-8230-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8230-5