Abstract
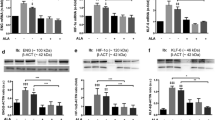
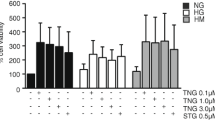
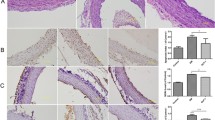
Recently, it has been demonstrated that Glucagon-like peptide-1 (GLP-1) has a protective effect on endothelial cells. Our hypothesis is that this GLP-1 protective effect is partly lost when the cells are exposed to sustained high glucose concentrations. Human umbilical vein endothelial cells (HUVECs) were cultured for 21 days in normal glucose (5 mmol/L, NG) or high glucose (25 mmol/L glucose, HG). GLP-1 (7-37) and Ruboxistaurin were added at 50 and 500 nM, respectively, alone or in combination, 1 h before cell harvesting. Analysis of GLP-1 receptor protein levels, as well as of the gene expression of different ER stress-related genes, proliferation markers, antioxidant cell response-related genes, and PKA subunits, was performed. ROS production was also measured in HUVECs exposed to mentioned treatments. GLP-1 receptor expression was reduced in HUVECs exposed to chronic high glucose concentrations but was partially restored by a chemical PKCβ-specific inhibitor. GLP-1, added as an acute treatment in endothelial cells, had the capacity to induce the expression of Nrf2-detoxifying enzyme targets, to increase transcription levels of scavenger genes, to attenuate the expression of high glucose-induced PKA subunits, ER stress and also the apoptotic phenotype of HUVECs; these effects occured only when high glucose-induced PKCβ overexpression was reduced by Ruboxistaurin. In a similar manner, ROS production induced by high glucose was reduced by GLP-1 in the presence of PKCβ inhibitor. This study suggests that an increase in PKCβ, induced by high glucose, could have a role in endothelial GLP-1 resistance, reducing GLP-1 receptor levels and disrupting the GLP-1 canonical pathway.







Similar content being viewed by others
References
S. Herzberg-Schafer, M. Heni, N. Stefan, H.U. Haring, A. Fritsche, Impairment of GLP1-induced insulin secretion: role of genetic background, insulin resistance and hyperglycaemia. Diabetes Obes. Metab. 14(Suppl 3), 85–90 (2012)
M. Nauck, F. Stockmann, R. Ebert, W. Creutzfeldt, Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29, 46–52 (1986)
D.J. Drucker, The biology of incretin hormones. Cell Metab. 3, 153–165 (2006)
Y. Ishibashi, T. Matsui, M. Takeuchi, S. Yamagishi, Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem. Biophys. Res. Commun. 391, 1405–1408 (2010)
L. Ding, J. Zhang, Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol. Sin. 33, 75–81 (2012)
G.G. Holz, Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 53, 5–13 (2004)
K. Ban, M.H. Noyan-Ashraf, J. Hoefer, S.S. Bolz, D.J. Drucker, M. Husain, Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117, 2340–2350 (2008)
T. Nystrom, M.K. Gutniak, Q. Zhang, F. Zhang, J.J. Holst, B. Ahren, A. Sjoholm, Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am. J. Physiol. Endocrinol. Metab. 287, E1209–E1215 (2004)
A. Ceriello, K. Esposito, R. Testa, A.R. Bonfigli, M. Marra, D. Giugliano, The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care 34, 697–702 (2011)
H. Oeseburg, R.A. de Boer, H. Buikema, P. van der Harst, W.H. van Gilst, H.H. Sillje, Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler. Thromb. Vasc. Biol. 30, 1407–1414 (2010)
A. Pecorelli, V. Bocci, A. Acquaviva, G. Belmonte, C. Gardi, F. Virgili, L. Ciccoli, G. Valacchi, NRF2 activation is involved in ozonated human serum upregulation of HO-1 in endothelial cells. Toxicol. Appl. Pharmacol. 267, 30–40 (2013)
J. Liu, Y. Liu, L. Chen, Y. Wang, J. Li, Glucagon-Like Peptide-1 Analog Liraglutide Protects against Diabetic Cardiomyopathy by the Inhibition of the Endoplasmic Reticulum Stress Pathway. J. Diabetes Res. 2013, 630537 (2013)
B. Schisano, A.L. Harte, K. Lois, P. Saravanan, N. Al-Daghri, O. Al-Attas, L.B. Knudsen, P.G. McTernan, A. Ceriello, G. Tripathi, GLP-1 analogue, Liraglutide protects human umbilical vein endothelial cells against high glucose induced endoplasmic reticulum stress. Regul. Pept. 174, 46–52 (2012)
L. Zhao, H. Guo, H. Chen, R.B. Petersen, L. Zheng, A. Peng, K. Huang, Effect of Liraglutide on endoplasmic reticulum stress in diabetes. Biochem. Biophys. Res. Commun. 441, 133–138 (2013)
A. Mima, J. Hiraoka-Yamomoto, Q. Li, M. Kitada, C. Li, P. Geraldes, M. Matsumoto, K. Mizutani, K. Park, C. Cahill, S. Nishikawa, C. Rask-Madsen, G.L. King, Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCbeta activation in diabetes. Diabetes 61, 2967–2979 (2012)
T.P. Solomon, S.H. Knudsen, K. Karstoft, K. Winding, J.J. Holst, B.K. Pedersen, Examining the effects of hyperglycemia on pancreatic endocrine function in humans: evidence for in vivo glucotoxicity. J. Clin. Endocrinol. Metab. 97, 4682–4691 (2012)
C.J. Green, T.I. Henriksen, B.K. Pedersen, T.P. Solomon, Glucagon like peptide-1-induced glucose metabolism in differentiated human muscle satellite cells is attenuated by hyperglycemia. PLoS One 7, e44284 (2012)
G. Xu, H. Kaneto, D.R. Laybutt, V.F. Duvivier-Kali, N. Trivedi, K. Suzuma, G.L. King, G.C. Weir, S. Bonner-Weir, Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes 56, 1551–1558 (2007)
C. Gorrini, I.S. Harris, T.W. Mak, Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 (2013)
A.C. Brewer, T.V. Murray, M. Arno, M. Zhang, N.P. Anilkumar, G.E. Mann, A.M. Shah, Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic. Biol. Med. 51, 205–215 (2011)
D.J. Drucker, Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol. Endocrinol. 17, 161–171 (2003)
T. Forst, M.M. Weber, A. Pfutzner, Cardiovascular benefits of GLP-1-based therapies in patients with diabetes mellitus type 2: effects on endothelial and vascular dysfunction beyond glycemic control. Exp. Diabetes Res. 2012, 635472 (2012)
K. Ban, S. Hui, D.J. Drucker, M. Husain, Cardiovascular consequences of drugs used for the treatment of diabetes: potential promise of incretin-based therapies. J. Am. Soc. Hypertens. 3, 245–259 (2009)
D. Accili, K.C. Arden, FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421–426 (2004)
K. Soberg, T. Jahnsen, T. Rognes, B.S. Skalhegg, J.K. Laerdahl, Evolutionary paths of the cAMP-dependent protein kinase (PKA) catalytic subunits. PLoS One 8, e60935 (2013)
S. Rajan, L.M. Dickson, E. Mathew, C.M. Orr, J.H. Ellenbroek, L.H. Philipson, B. Wicksteed, Chronic hyperglycemia downregulates GLP-1 receptor signaling in pancreatic beta-cells via protein kinase A. Mol. Metab. 4, 265–276 (2015)
T.W. Kensler, N. Wakabayashi, S. Biswal, Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47, 89–116 (2007)
M. Kobayashi, L. Li, N. Iwamoto, Y. Nakajima-Takagi, H. Kaneko, Y. Nakayama, M. Eguchi, Y. Wada, Y. Kumagai, M. Yamamoto, The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 29, 493–502 (2009)
M. Kobayashi, M. Yamamoto, Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 7, 385–394 (2005)
M. Kobayashi, M. Yamamoto, Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 46, 113–140 (2006)
C.Y. Tsai, C.C. Wang, T.Y. Lai, H.N. Tsu, C.H. Wang, H.Y. Liang, W.W. Kuo, Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int. J. Cardiol. 168, 1286–1297 (2013)
S.G. Fonseca, M. Burcin, J. Gromada, F. Urano, Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr. Opin. Pharmacol. 9, 763–770 (2009)
L. Xu, G.A. Spinas, M. Niessen, ER stress in adipocytes inhibits insulin signaling, represses lipolysis, and alters the secretion of adipokines without inhibiting glucose transport. Horm. Metab. Res. 42, 643–651 (2010)
C.J. van der Kallen, M.M. van Greevenbroek, C.D. Stehouwer, C.G. Schalkwijk, Endoplasmic reticulum stress-induced apoptosis in the development of diabetes: is there a role for adipose tissue and liver? Apoptosis Int. J. Program. Cell Death 14, 1424–1434 (2009)
S. Alhusaini, K. McGee, B. Schisano, A. Harte, P. McTernan, S. Kumar, G. Tripathi, Lipopolysaccharide, high glucose and saturated fatty acids induce endoplasmic reticulum stress in cultured primary human adipocytes: Salicylate alleviates this stress. Biochem. Biophys. Res. Commun. 397, 472–478 (2010)
C.S. McAlpine, A.J. Bowes, G.H. Werstuck, Diabetes, hyperglycemia and accelerated atherosclerosis: evidence supporting a role for endoplasmic reticulum (ER) stress signaling. Cardiovasc. Hematol. Disord. 10, 151–157 (2010)
W. Bakker, E.C. Eringa, P. Sipkema, V.W. van Hinsbergh, Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 335, 165–189 (2009)
J. Lee, S.W. Hong, S.E. Park, E.J. Rhee, C.Y. Park, K.W. Oh, S.W. Park, W.Y. Lee, Exendin-4 attenuates endoplasmic reticulum stress through a SIRT1-dependent mechanism. Cell Stress Chaperones 19, 649–656 (2014)
C.W. Younce, M.A. Burmeister, J.E. Ayala, Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. Am. J. Physiol. Cell Physiol. 304, C508–C518 (2013)
B. Yusta, L.L. Baggio, J.L. Estall, J.A. Koehler, D.P. Holland, H. Li, D. Pipeleers, Z. Ling, D.J. Drucker, GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 4, 391–406 (2006)
S. Tsunekawa, N. Yamamoto, K. Tsukamoto, Y. Itoh, Y. Kaneko, T. Kimura, Y. Ariyoshi, Y. Miura, Y. Oiso, I. Niki, Protection of pancreatic beta-cells by exendin-4 may involve the reduction of endoplasmic reticulum stress; in vivo and in vitro studies. J. Endocrinol. 193, 65–74 (2007)
M. Shimoda, Y. Kanda, S. Hamamoto, K. Tawaramoto, M. Hashiramoto, M. Matsuki, K. Kaku, The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia 54, 1098–1108 (2011)
B. Basha, S.M. Samuel, C.R. Triggle, H. Ding, Endothelial dysfunction in diabetes mellitus: possible involvement of endoplasmic reticulum stress? Exp. Diabetes Res. 2012, 481840 (2012)
Z. Zhang, J. Li, X. Jiang, L. Yang, L. Lei, D. Cai, H. Zhang, H. Chen, GLP-1 ameliorates the proliferation activity of INS-1 cells inhibited by intermittent high glucose concentrations through the regulation of cyclins. Mol. Med. Rep. 10, 683–688 (2014)
Y.H. Cheong, M.K. Kim, M.H. Son, B.K. Kaang, Glucose exposure pattern determines glucagon-like peptide 1 receptor expression and signaling through endoplasmic reticulum stress in rat insulinoma cells. Biochem. Biophys. Res. Commun. 414, 220–225 (2011)
Q.R. Pan, W.H. Li, H. Wang, Q. Sun, X.H. Xiao, B. Brock, O. Schmitz, Glucose, metformin, and AICAR regulate the expression of G protein-coupled receptor members in INS-1 beta cell. Horm. Metab. Res. 41, 799–804 (2009)
I. Valverde, G.S. Wang, K. Burghardt, L.M. Kauri, A. Redondo, A. Acitores, M.L. Villanueva-Penacarrillo, P. Courtois, A. Sener, J. Cancelas, W.J. Malaisse, F.W. Scott, Bioactive GLP-1 in gut, receptor expression in pancreas, and insulin response to GLP-1 in diabetes-prone rats. Endocrine 23, 77–84 (2004)
Acknowledgments
We thank Dr. Díaz for providing us with HUVECs, and Kimberly Katte of the Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) for assisting with manuscript editing.
Funding
This study was supported by project PI10/01256 within the framework of the Plan Nacional de I+D+I and co-funded by the Carlos III Health Institute (ISCIII)-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER), Spain.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pujadas, G., De Nigris, V., La Sala, L. et al. The pivotal role of high glucose-induced overexpression of PKCβ in the appearance of glucagon-like peptide-1 resistance in endothelial cells. Endocrine 54, 396–410 (2016). https://doi.org/10.1007/s12020-015-0799-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0799-z