Abstract
Introduction
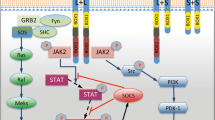
Prolactin is a peptide hormone mainly synthetized and secreted by the anterior pituitary gland, but also by extrapituitary tissues, such as mammary gland, decidua, prostate, skin, and possibly the brain. Similarly, prolactin receptor is expressed in the pituitary gland, many peripheral tissues, and in contrast to prolactin, its receptor has been consistently detected in several brain regions, such as cerebral cortex, olfactory bulb, hypothalamus, hippocampus, amygdala, among others. Classically, prolactin function has been related to the stimulation of lactogenesis and galactopoiesis, however, it is well known that prolactin induces a wide range of functions in different brain areas.
Purpose
The aim of this review is to summarize recent reports on prolactin and prolactin receptor synthesis and localization, as well as recapitulate both the classic functions attributed to this hormone in the brain and the recently described functions such as neurogenesis, neurodevelopment, sleep, learning and memory, and neuroprotection.
Conclusion
The distribution and putative expression of prolactin and its receptors in several neuronal tissues suggests that this hormone has pleiotropic functions in the brain.

Similar content being viewed by others
References
A. Ignacak, M. Kasztelnik, T. Sliwa, R.A. Korbut, K. Rajda, T.J. Guzik, Prolactin—Not only lactotrophin a “new” view of the “old” hormone. J. Physiol. Pharmacol. 63(5), 435–443 (2012)
R.J. Marano, N. Ben-Jonathan, Minireview: extrapituitary prolactin: an update on the distribution, regulation, and functions. Mol. Endocrinol. 28(5), 622–633 (2014)
D.R. Grattan, I.C. Kokay, Prolactin: a pleiotropic neuroendocrine hormone. J. Neuroendocrinol. 20(6), 752–763 (2008)
R., Bates, E., Lahr, O., Riddle, The gross action of prolactin and follicle-stimulating hormone on the mature ovary and sex accessories of fowl. Am. J. Physiol. I(II), 361–368 (1935)
N. Ben-jonathan, J.L. Mershonf, D.L. Allen, R.W. Steinmetz, Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr. Rev. 17(6), 639–669 (1996)
S. Harvey, Extrapituitary growth hormone. Endocrine 38(3), 335–359 (2010)
S. Harvey, C. Arámburo, E.J. Sanders, Extrapituitary production of anterior pituitary hormones: an overview. Endocrine 41(1), 19–30 (2012)
R.J., Walsh, F.J., Slaby, B.I.Posner, A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology 120(5), 1846–1850 (1987)
R.S.E. Brown, A.K. Wyatt, R.E. Herbison, P.J. Knowles, S.R. Ladyman, N. Binart, W.A. Banks, D.R. Grattan, Prolactin transport into mouse brain is independent of prolactin receptor. FASEB J. 30(2), 1002–1010 (2016)
N. Emanuele, J. Jurgens, M. Halloran, J. Tentler, A. Lawrence, M. Kelley, The rat prolactin gene is expressed in brain tissue: detection of normal and alternatively spliced prolactin messenger RNA. Mol. Endocrinol. 6(1), 35–42 (1992)
K. Fields, E. Kulig, R. Lloyd, Detection of prolactin messenger RNA in mammary and other normal and neoplastic tissues by polymerase chain reaction. Lab. Invest. 68(3), 354–360 (1993)
H. Ehmann, C. Salzig, P. Lang, E. Friauf, H. Nothwang, Minimal sex differences in gene expression in the rat superior olivary complex. Hear. Res. 245(1–2), 65–72 (2008)
W. DeVito, C. Avakian, S. Stone, C. Ace, Estradiol increases prolactin synthesis and prolactin messenger ribonucleic acid in selected brain regions in the hypophysectomized female rat. Endocrinology 131(5), 2154–2160 (1992)
C.E. Roselli, S. Bocklandt, H.L. Stadelman, T. Wadsworth, E. Vilain, F. Stormshak, Prolactin expression in the sheep brain. Neuroendocrinology 87(4), 206–215 (2008)
W. DeVito, Distribution of immunoreactive prolactin in the male and female rat brain: effects of hypophysectomy and intraventricular administration of colchicine. Neuroendocrinology 47(4), 284–289 (1988)
V. Goffin, P. Kelly, The prolactin/growth hormone receptor family: structure/function relationships. J. Mammary Gland Biol. Neoplasia 2(1), 7–17 (1997)
C. Bole-Feysot, V. Goffin, M. Edery, N. Binart, P. Kelly, Prolactin (PRL) and its receptor: action signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19(3), 225–268 (1998)
M. Freeman, B. Kanyicska, A. Lenart, G. Nagy, Prolactin: structure, function and regulation of secretion. Physiol. Rev. 80, 1523–1631 (2000)
C. Clevenger, J. Kline, Prolactin receptor signal transduction. Lupus 10(10), 706–718 (2001)
X. Pi, J.L. Voogt, Sex difference and estrous cycle: Expression of prolactin receptor mRNA in rat brain. Mol. Brain Res. 103(1–2), 130–139 (2002)
J. Bakowska, J. Morrell, The distribution of mRNA for the short form of the prolactin receptor in the forebrain of the female rat. Brain Res. 116(1–2), 50–58 (2003)
J.C. Bakowska, J.I. Morrell, Atlas of the neurons that express mRNA for the long form of the prolactin receptor in the forebrain of the female rat. J. Comp. Neurol. 386(2), 161–177 (1997)
X.J. Pi, D.R. Grattan, Increased expression of both short and long forms of prolactin receptor mRNA in hypothalamic nuclei of lactating rats. J. Mol. Endocrinol. 23(1), 13–22 (1999)
T. Fujikawa, H. Soya, H. Yoshizato, K. Sakaguchi, K. Doh-Ura, M. Tanaka, K. Nakashima, Restraint stress enhances the gene expression of prolactin receptor long form at the choroid plexus. Endocrinology 136, 5608–5613 (1995)
J. Hirai, M. Nishita, N. Nakao, T.R. Saito, M. Tanaka, Regulation of prolactin receptor gene expression in the rat choroid plexus via transcriptional activation of multiple first exons during postnatal development and lactation. Exp. Anim. 62(1), 49–56 (2013)
S. Chiu, P.M. Wise, Prolactin receptor mRNA localization in the hypothalamus by in situ hybridization. J. Neuroendocrinol. 6(2), 191–199 (1994)
R.S. Brown, I.C. Kokay, A.E. Herbison, D.R. Grattan, Distribution of prolactin-responsive neurons in the mouse forebrain. J. Comp. Neurol. 518(1), 92–102 (2010)
T.A. Möderscheim, T. Gorba, P. Pathipati, I.C. Kokay, D.R. Grattan, C.E. Williams, A. Scheepens, Prolactin is involved in glial responses following a focal injury to the juvenile rat brain. Neuroscience 145(3), 963–973 (2007)
L. Torner, S. Karg, A. Blume, M. Kandasamy, H.G. Kuhn, J. Winkler, L. Aigner, I.D. Neumann, Prolactin prevents chronic stress-induced decrease of adult hippocampal neurogenesis and promotes neuronal fate. J. Neurosci. 29(6), 1826–1833 (2009)
E.A. Cabrera-Reyes, E. Vergara-Castañeda, N. Rivero-Segura, M. Cerbón, Sex differences in prolactin and its receptor expression in pituitary, hypothalamus, and hippocampus of the rat. Rev. Mex. Endocrinol. Metab. Nutr. 2(2), 60–67 (2015)
X.J. Pi, D.R. Grattan, Distribution of prolactin receptor immunoreactivity in the brain of estrogen-treated, ovariectomized rats. J. Comp. 394, 462–474 (1998)
Allen Institute for Brain Science, Allen Mouse Brain Atlas. http://mouse.brain-map.org/experiment/show/72340223 (2004).
M. Shamgochian, C. Avakian, N. Truong, S. Stone, K. Tang, W. DeVito, Regulation of prolactin receptor expression by estradiol in the female rat brain. Neuroreport. 6(18), 2537–2541 (1995)
P. Mann, R. Bridges, Prolactin receptor gene expression in the forebrain of pregnant and lactating rats. Brain Res. Mol. Brain Res. 105(1–2), 136–145 (2002)
S. Chiu, R.D. Koos, P.M. Wise, Detection of prolactin receptor (PRL-R) mRNA in the rat hypothalamus and pituitary gland. Endocrinology 130(3), 1747–1749 (1992)
S.H. Yip, R. Eguchi, D.R. Grattan, S.J. Bunn, Prolactin signalling in the mouse hypothalamus is primarily mediated by signal transducer and activator of transcription factor 5b but not 5a. J. Neuroendocrinol. 24(12), 1484–1491 (2012)
R. Das, B. Vonderhaar, Transduction of prolactin’s (PRL) growth signal through both long and short forms of the PRL receptor. Mol. Endocrinol. 9, 1750–1759 (1995)
J. Harris, P.M. Stanford, S.R. Oakes, C.J. Ormandy, Prolactin and the prolactin receptor: new targets of an old hormone. Ann. Med. 36(6), 414–425 (2004)
L. Torner, E. Tinajero, N. Lajud, A. Quintanar-Stéphano, E. Olvera-Cortés, Hyperprolactinemia impairs object recognition without altering spatial learning in male rats. Behav. Brain Res. 252, 32–39 (2013)
M.J. Patil, M.A. Henry, A.N. Akopian, Prolactin receptor in regulation of neuronal excitability and channels. Channels 8(3), 193–202 (2014)
F. Drago, Prolactin and sexual behavior: a review. Neurosci. Biobehav. Rev. 8, 433–439 (1984)
D. Tejadilla, M. Cerbón, T. Morales, Prolactin reduces the damaging effects of excitotoxicity in the dorsal hippocampus of the female rat independently of ovarian hormones. Neuroscience 169(3), 1178–1185 (2010)
A. Vanoye-Carlo, T. Morales, E. Ramos, A. Mendoza-Rodríguez, M. Cerbón, Neuroprotective effects of lactation against kainic acid treatment in the dorsal hippocampus of the rat. Horm. Behav. 53(1), 112–123 (2008)
V. Cabrera, D. Cantú, E. Ramos, A. Vanoye-Carlo, M. Cerbón, T. Morales, Lactation is a natural model of hippocampus neuroprotection against excitotoxicity. Neurosci. Lett. 461(2), 136–139 (2009)
V. Cabrera, E. Ramos, A. González-Arenas, M. Cerbón, I. Camacho-Arroyo, T. Morales, Lactation reduces glial activation induced by excitotoxicity in the rat hippocampus. J. Neuroendocrinol. 25(6), 519–527 (2013)
R. Brown, A. Herbison, D. Grattan, Effects of prolactin and lactation on A15 dopamine neurones in the rostral preoptic area of female mice. J. Neuroendocrinol. 27(9), 708–717 (2015)
J.A. Seggie, G.M. Brown, Stress response patterns of plasma corticosterone, prolactin, and growth hormone in the rat, following handling or exposure to novel environment. Can. J. Physiol. Pharmacol. 53(4), 629–637 (1975)
A.C. de Moura, V.M. Lazzari, R.O. Becker, M.S. Gil, C.A. Ruthschilling, G. Agnes, S. Almeida, A.B. da Veiga, A.B. Luicion, M. Giovenardo, Gene expression in the CNS of lactating rats with different patterns of maternal behavior. Neurosci. Res. 99, 8–15 (2015)
C.M. Larsen, I.C. Kokay, D.R. Grattan, Male pheromones initiate prolactin-induced neurogenesis and advance maternal behavior in female mice. Horm. Behav. 53(4), 509–517 (2008)
F. Lévy, M. Keller, P. Poindron, Olfactory regulation of maternal behavior in mammals. Horm. Behav. 46(3), 284–302 (2004)
H. Salais-López, E. Lanuza, C. Agustín-Pavón, F. Martínez-García, Tuning the brain for motherhood: prolactin-like central signalling in virgin, pregnant, and lactating female mice. Brain Struct. Funct. 222(2), 895–921 (2017)
M. Freemark, P. Driscoll, J. Andrews, P.A. Kelly, M. Royster, Ontogenesis of prolactin receptor gene expression in the rat olfactory system: potential roles for lactogenic hormones in olfactory development. Endocrinology 137(3), 934–942 (1996)
T. Shingo, C. Gregg, E. Enwere, H. Fujikawa, R. Hassam, C. Geary, J.C. Cross, S. Weiss, Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299(5603), 117–120 (2003)
R. Bridges, D. Grattan, Prolactin-induced neurogenesis in the maternal brain. Trends Endocrinol. Metab. 14(5), 199–201 (2003)
N., Lajud, R., Gonzalez-Zapien, A., Roque, E., Tinajero, J.J., Valdez, C., Clapp, L., Torner, Prolactin administration during early postnatal life decreases hippocampal and olfactory bulb neurogenesis and results in depressive-like behavior in adulthood. Horm. Behav. 64(5), 781–789 (2013)
A. Fleming, M. Korsmit, Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav. Neurosci. 110(3), 567–582 (1996)
Y. Elyada, A. Mizrahi, Becoming a mother-circuit plasticity underlying maternal behavior. Curr. Opin. Neurobiol. 35, 49–56 (2015)
J.N. Lundström, A. Mathe, B. Schaal, J. Frasnelli, K. Nitzsche, J. Gerber, T. Hummel, Maternal status regulates cortical responses to the body odor of newborns. Front. Psychol. 4, 1–6 (2013)
L. Cohen, A. Mizrahi, Plasticity during motherhood: changes in excitatory and inhibitory layer 2/3 neurons in auditory cortex. J. Neurosci. 35(4), 1806–1815 (2015)
C. Gregg, V. Shikar, P. Larsen, G. Mak, A. Chojnacki, V.W. Yong, S. Weiss, White matter plasticity and enhanced remyelination in the maternal CNS. J. Neurosci. 27(8), 1812–1823 (2007)
M. Maheu, E. Akbari, A. Fleming, Callosal oligodendrocyte number in postpartum Sprague-Dawley rats. Brain Res. 24(1267), 18–24 (2009)
P. Mann, R. Bridges, Lactogenic hormone regulation of maternal behavior. Prog. Brain Res. 133, 251–262 (2001)
M. Numan, M. Numan, A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev. Psycobiol. 29(1), 23–51 (1996)
M. Numan, A neural circuitry analysis of maternal behavior in the rat. Acta Paediatr. Suppl. 397, 19–28 (1994)
A. Consiglio, A. Borsoi, G. Pereira, A. Lucion, Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol. Behav. 85(3), 354–362 (2005)
S. Kenny, L. Wright, A. Green, R. Mashoodh, T. Perrot, Expression of maternal behavior and activation of the bed nucleus of the stria terminalis during predatory threat exposure: modulatory effects of transport stress. Physiol. Behav. 17(123), 148–155 (2014)
L.P. Mangurian, R.J. Walsh, B.I. Posner, Prolactin enhancement of its own uptake at the choroid plexus. Endocrinology 131, 698–702 (1992)
R.A. Augustine, I.C. Kokay, Z.B. Andrews, S.R. Ladyman, D.R. Grattan, Quantitation of prolactin receptor mRNA in the maternal rat brain during pregnancy and lactation. J. Mol. Endocrinol. 31(1), 221–232 (2003)
H. Tabata, M. Kobayashi, J.H. Ikeda, N. Nakao, T.R. Saito, M. Tanaka, Characterization of multiple first exons in murine prolactin receptor gene and the effect of prolactin on their expression in the choroid plexus. J. Mol. Endocrinol. 48(2), 169–176 (2012)
X.J. Pi, D.R. Grattan, Differential expression of the two forms of prolactin receptor mRNA within microdissected hypothalamic nuclei of the rat. Brain Res. Mol. Brain Res. 59(1), 1–12 (1998)
R. Abs, E. Van Vleymen, P. Parizel, K. Van Acker, M. Martin, J. Martin, Congenital cerebellar hypoplasia and hypogonadotropic hypogonadism. J. Neurol. Sci. 98(2–3), 259–265 (1990)
G. Leng, R. Dyball, S. Luckman, Mechanisms of vasopressin secretion. Horm. Res. 37(1–2), 33–38 (1992)
G. Kennedy, The hypothalamic control of food intake in rats. Proc. R. Soc. Lond. B. Biol. Sci. 137(889), 535–549 (1950)
C.M., Brooks, E., Lambert, P., Bard, Experimental production of obesity in the monkey (Macaca mulatta). Fed. Proc. 1, 11 (1942)
D. Sauvé, B. Woodside, The effect of central administration of prolactin on food intake in virgin female rats is dose-dependent, occurs in the absence of ovarian hormones and the latency to onset varies with feeding regimen. Brain Res. 729(1), 75–81 (1996)
D. Sauve, B. Woodside, Neuroanatomical specificity of prolactin-induced hyperphagia in virgin female rats. Brain Res. 868(2), 306–314 (2000)
S. Mejía, L.M. Torner, M.C. Jeziorski, C. Gonzalez, M.A. Morales, G.M. Martínez de la Escalera, C.: Clapp, Prolactin and 16K prolactin stimulate release of vasopressin by a direct effect on hypothalamo-neurohypophyseal system. Endocrine 20(1–2), 155–162 (2003)
S. Mejía, M. Morales, M. Zetina, G. Martínez de la Escalera, C.: Clapp, Immunoreactive prolactin forms colocalize with vasopressin in neurons of the hypothalamic paraventricular and supraoptic nuclei. Neuroendocrinology 66(3), 151–159 (1997)
C. Pedersen, J. Ascher, Y. Monroe, A.J. Prange, Oxytocin induces maternal behavior in virgin female rats. Science 216(4546), 648–650 (1982)
J.M. Zimmermann-Peruzatto, V.M. Lazzari, A.C. de Moura, S. Almeida, M. Giovenardi, Examining the role of vasopressin in the modulation of parental and sexual behaviors. Front. Psychiatry 6, 1–8 (2015)
H.H. Van Tol, E.L. Bolwerk, B. Liu, J.P. Burbach, Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology 122(3), 945–951 (1988)
N. Donner, I. Neumann, Effects of chronic intracerebral prolactin on the oxytocinergic and vasopressinergic system of virgin ovariectomized rats. Neuroendocrinology 90(3), 315–322 (2009)
R., Augustine, G., Bouwer, A., Seymour, D., Grattan, C., Brown, Reproductive regulation of gene expression in the hypothalamic supraoptic and paraventricular nuclei. J. Neuroendocrinol. (4), (2016). doi:10.1111/jne.12350
K. Suzuki, N. Koizumi, H. Hirose, R. Hokao, N. Takemura, S. Motoyoshi, Changes in plasma arginine vasopressin concentration during lactation in rats. Comp. Med. 50(3), 277–280 (2000)
N. Donner, R. Bredewold, R. Maloumby, I.D. Neumann, Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur. J. Neurosci. 25(6), 1804–1814 (2007)
L. Krulich, E. Hefco, P. Illner, C.B. Read, The effects of acute stress on the secretion of LH, FSH, prolactin and GH in the normal male rat, with comments on their statistical evaluation. Neuroendocrinology 16, 293–311 (1974)
B. Bodosi, F. Obál Jr., J. Gardi, J. Komlódi, J. Fang, J.M. Krueger, An ether stressor increases REM sleep in rats: possible role of prolactin. Am. J. Physiol. 279(5), 1590–1589 (2000)
R.R. Gala, The physiology and mechanisms of the stress-induced changes in prolactin secretion in the rat. Life Sci. 46, 1407–1420 (1990)
F. Obal Jr., M. Opp, A.B. Cady, L. Johannsen, J.M. Krueger, Prolactin, vasoactive intestinal peptide, and peptide histidine methionine elicit selective increases in REM sleep in rabbits. Brain Res. 490, 292–300 (1989)
P. Meerlo, A. Easton, B.M. Bergmann, F.W. Turek, Restraint increases prolactin and REM sleep in C57BL/6J mice but not in BALB/cJ mice. Am. J. Physiol. 281(3), 846–854 (2001)
R.B. Machado, M.R. Rocha, D. Suchecki, Brain prolactin is involved in stress-induced REM sleep rebound. Horm. Behav. 89, 38–47 (2016)
R.M. Frieboes, H. Murck, G.K. Stalla, I.A. Antonijevic, A. Steiger, Enhanced slow wave sleep in patients with prolactinoma. J. Clin. Endocrinol. Metab. 83, 2706–2710 (1998)
K. Koike, A. Miyake, T. Aono, T. Sakumoto, M. Ohmichi, M. Yamaguchi, O. Tanizawa, Effect of prolactin on the secretion of hypothalamic GnRH and pituitary gonadotropins. Horm. Res. 35(1), 5–12 (1991)
R. Araujo-Lopes, J. Crampton, N. Aquino, R. Miranda, I. Kokay, A. Reis, C.R. Franci, D.R. Grattan, R.E. Szawka, Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology 155(3), 1010–1020 (2014)
J. Clarkson, X. d’Anglemont de Tassigny, W. Colledge, A. Caraty, A. Herbison, Distribution of kisspeptin neurones in the adult female mouse brain. J. Neuroendocrinol. 21(8), 673–682 (2009)
I.C. Kokay, S.L. Petersen, D.R. Grattan, Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology 152(2), 526–535 (2011)
I. Cohen-Becker, M. Selmanoff, P. Wise, Hyperprolactinemia alters the frequency and amplitude of pulsatile luteinizing hormone secretion in the ovariectomized rat. Neuroendocrinology 42(4), 328–333 (1986)
A. Lerant, M.E. Freeman, Ovarian steroids differentially regulate the expression of PRL-R in neuroendocrine dopaminergic neuron populations: A double label confocal microscopic study. Brain Res. 802(1–2), 141–154 (1998)
G. Anderson, D. Grattan, W. van den Ancker, R. Bridges, Reproductive experience increases prolactin responsiveness in the medial preoptic area and arcuate nucleus of female rats. Endocrinology 147, 4688–4694 (2006)
L. Torner, R. Maloumby, G. Nava, J. Aranda, C. Clapp, I.D. Neumann, In vivo release and gene upregulation of brain prolactin in response to physiological stimuli. Eur. J. Neurosci. 19(6), 1601–1608 (2004)
J.A. McHenry, D.R. Rubinow, G.D. Stuber, Maternally responsive neurons in the bed nucleus of the stria terminalis and medial preoptic area: Putative circuits for regulating anxiety and reward. Front. Neuroendocrinol. 38, 65–72 (2015)
R.S. Bridges, M. Numan, P.M. Ronsheim, P.E. Mann, C.E. Lupini, Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc. Natl. Acad. Sci. USA 87(20), 8003–8007 (1990)
F.K. Stephan, I. Zucker, Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 69(6), 1583–1586 (1972)
C. Bethea, J. Neill, Lesions of the suprachiasmatic nuclei abolish the cervically stimulated prolactin surges in the rat. Endocrinology 107(1), 1–5 (1980)
K. Brown-Grant, G. Raisman, Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc. R. Soc. Lond. B Biol. Sci. 198(1132), 279–296 (1977)
J.T. Pan, R.R. Gala, Central nervous system regions involved in the estrogen-induced afternoon prolactin surge. II. Implantation studies. Endocrinology 117(1), 388–395 (1985)
J. Pan, R. Gala, Central nervous system regions involved in the estrogen-induced afternoon prolactin surge. I. Lesion studies. Endocrinology 117(1), 382–387 (1985)
I. Clarke, J. Cummins, D. de Kretser, Pituitary gland function after disconnection from direct hypothalamic influences in the sheep. Neuroendocrinology 36(5), 376–384 (1983)
J.D. Johnston, D.J. Skene, Regulation of mammalian neuroendocrine physiology and rhythms by melatonin. J. Endocrinol. 226(2), T187–T198 (2015)
S. Shyr, W. Crowley, C. Grosvenor, Effect of neonatal prolactin deficiency on prepubertal tuberoinfundibuler and tuberohypophyseal dopaminergic neuronal activity. Endocrinology 119(3), 1217–1221 (1986)
C. Grosvenor, N. Whitworth, Accumulation of prolactin in maternal milk and its transfer to circulation of neonate rat: a review. Endocrinol. Exp. 17, 271–282 (1983)
M. Royster, P. Driscoll, P. Kelly, M. Freemark, The prolactin receptor in the fetal rat: cellular localization of messenger ribonucleic acid, immunoreactive protein, and ligand-binding activity and induction of expression in late gestation. Endocrinology 136(9), 3892–3900 (1995)
A.I. Melo, M. Perez-Ledezma, C. Clapp, E. Arnold, J.C. Rivera, A.S. Fleming, Effects of prolactin deficiency during the early postnatal period on the development of maternal behavior in female rats: Mother’s milk makes the difference. Horm. Behav. 56(3), 281–291 (2009)
E. Vergara-Castañeda, D.R. Grattan, H. Pasantes-Morales, M. Pérez-Domínguez, E.A. Cabrera-Reyes, T. Morales, M. Cerbón, Prolactin mediates neuroprotection against excitotoxicity in primary cell cultures of hippocampal neurons via its receptor. Brain Res. 1636, 192–199 (2016)
N.V. Emanuele, L. Metcalfe, L. Wallock, J. Tentler, T.C. Hagen, C.T. Beer, D. Martinson, P.W. Gout, L. Kirsteins, A.M. Lawrence, Extrahyponthalamic brain prolactin: characterization and evidence for independence from pituitary prolactin. Brain Res. 421(1–2), 255–262 (1987)
H. Eichenbaum, Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44(1), 109–120 (2004)
G. Love, N. Torrey, I. McNamara, M. Morgan, M. Banks, N. Hester, E.R. Glasper, A.C. Devries, C.H. Kinsley, K.G. Lambert, Maternal experience produces long-lasting behavioral modifications in the rat. Behav. Neurosci. 119(4), 1084–1096 (2005)
C.H. Kinsley, L. Madonia, G.W. Gifford, K. Tureski, G.R. Griffin, C. Lowry, J. Williams, J. Collins, H. McLearie, K.G. Lambert, Motherhood improves learning and memory. Nature 402(6758), 137–138 (1999)
J.L. Pawluski, B.L. Vanderbyl, K. Ragan, L.A. Galea, First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or “mothering” alone. Behav. Brain Res. 175(1), 157–165 (2006)
J.L. Pawluski, S.K. Walker, L.A. Galea, Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm. Behav. 49(2), 143–149 (2006)
J. Henry, B. Sherwin, Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav. Neurosci. 126(1), 73–85 (2012)
T. Castanho, P. Moreira, C. Portugal-Nunes, A. Novais, P. Costa, J. Palha, N. Sousa, N.C. Santos, The role of sex and sex-related hormones in cognition, mood and well-being in older men and women. Biol. Psychol. 103, 158–166 (2014)
T.L. Walker, J. Vukovic, M.M. Koudijs, D.G. Blackmore, E.W. Mackay, A.M. Sykes, R.W. Overall, A.S. Hamlin, P.F. Bartlett, Prolactin stimulates precursor cells in the adult mouse hippocampus. PLoS One 7(9), 1–11 (2012)
J. Reyes-Mendoza, T. Morales, Post-treatment with prolactin protects hippocampal CA1 neurons of the ovariectomized female rat against kainic acid-induced neurodegeneration. Neuroscience 328, 58–68 (2016)
J.L. Pawluski, L.A. Galea, Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience 149(1), 53–67 (2007)
G. Mak, S. Weiss, Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat. Neurosci. 13(6), 753–758 (2010)
G.V. Pardo, J.F. Goularte, A.L. Hoefel, A.L. de Castro, L.C. Kucharski, A. da Rosa Araujo, A.B.: Lucion, Effects of sleep restriction during pregnancy on the mother and fetuses in rats. Physiol. Behav. 1(155), 66–76 (2016)
G. Mak, E. Enwere, C. Gregg, T. Pakarainen, M. Poutanen, I. Huhtaniemi, S. Weiss, Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat. Neurosci. 10(8), 1003–1011 (2007)
E. Gould, B.S. McEwen, P. Tanapat, L.A. Galea, E. Fuchs, Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 17(7), 2492–2498 (1997)
R.S. Duman, J. Malberg, S. Nakagawa, Regulation of adult neurogenesis by psychotropic drugs and stress. J. Pharmacol. Exp. Ther. 299(2), 401–407 (2001)
E.A. Nimchinsky, B.L. Sabatini, K. Svoboda, Structure and function of dendritic spines. Annu. Rev. Physiol. 64, 313–353 (2001)
C.H. Kinsley, R. Trainer, G. Stafisso-Sandoz, P. Quadros, L.K. Marcus, C. Hearon, E.A. Meyer, N. Hester, M. Morgan, F.J. Kozub, K.G. Lambert, Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm. Behav. 49(2), 131–142 (2006)
J.L. Pawluski, L.A. Galea, Hippocampal morphology is differentially affected by reproductive experience in the mother. J. Neurobiol. 66(1), 71–81 (2006)
J. Brusco, R. Wittmann, M. de Azevedo, A. Lucion, C. Franci, M. Giovenardi, A.A. Rasia-Filho, Plasma hormonal profiles and dendritic spine density and morphology in the hippocampal CA1 stratum radiatum, evidenced by light microscopy, of virgin and postpartum female rats. Neurosci. Lett. 438(3), 346–350 (2008)
R. Abbud, G. Hoffman, M. Smith, Cortical refractoriness to N-methyl-D,L-aspartic acid (NMDA) stimulation in the lactating rat: recovery after pup removal and blockade of progesterone receptors. Brain Res. 604(1–2), 16–23 (1993)
C.A. Standley, N-methyl-D-aspartate receptor binding is altered and seizure potential reduced in pregnant rats. Brain Res. 844(1–2), 10–19 (1999)
P. Anborgh, C. Godin, M. Pampillo, G. Dhami, L. Dale, S. Cregan, R. Truant, S.S. Ferguson, Inhibition of metabotropic glutamate receptor signaling by the huntingtin-binding protein optineurin. J. Biol. Chem. 280(41), 34840–34848 (2005)
M.R. Hynd, H.L. Scott, P.R. Dodd, Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 45(5), 583–595 (2004)
M. Doretto, M. Oliveira-e-Silva, D. Ferreira-Alves, S. Pires, N. Garcia-Cairasco, A. Reis, Effect of lactation on the expression of audiogenic seizures: association with plasma prolactin profiles. Epilepsy Res. 54, 109–121 (2003)
P. Berzaghi Mda, D. Amado, E. Cavalheiro, Pregnancy decreases the frequency of spontaneous recurrent seizures in rats with kainic acid lesions of the hippocampus. Epilepsy Res. 1(2), 142–144 (1987)
T. Morales, M. Lorenson, A.M. Walker, E. Ramos, Both prolactin (PRL) and a molecular mimic of phosphorylated PRL, S179D-PRL, protect the hippocampus of female rats against excitotoxicity. Neuroscience 258, 211–217 (2014)
N.A. Rivero-Segura, E. Flores-Soto, S. García de la Cadena, I. Coronado-Mares, J.C. Gomez-Verjan, D.G. Ferreira, E.A. Cabrera-Reyes, L.V. Lopes, L. Massieu, M.: Cerbón, Prolactin-induced neuroprotection against glutamate excitotoxicity is mediated by the reduction of [Ca2+]i overload and NF-κB activation. PLoS One 12(5), e0176910 (2017)
A.M. Thomson, Neocortical layer 6, a review. Front. Neuroanat. 4, 13 (2010)
I.Y. Zhou, R.W. Chan, L.C. Ho, E.X. Wu, Longitudinal metabolic changes in the hippocampus and thalamus of the maternal brain revealed by proton magnetic resonance spectroscopy. Neurosci. Lett. 553, 170–175 (2013)
J. Broida, S.D. Michael, B. Svare, Plasmin prolactin levels are not related to the initiation, maintenance, and decline of postpartum aggression in mice. Behav. Neural. Biol. 32(1), 121–125 (1981)
M. Garland, B. Svare, Suckling stimulation modulates the maintenance of postpartum aggression in mice. Physiol. Behav. 44(3), 301–305 (1988)
O.J. Bosch, I.D. Neumann, Vasopressin released within the central amygdala promotes maternal aggression. Eur. J. Neurosci. 31(5), 883–891 (2010)
M. Davis, The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15, 353–375 (1992)
J. Allen, C. Allen, Role of the amygdaloid complexes in the stress-induced release of ACTH in the rat. Neuroendocrinology 15(3–4), 220–230 (1974)
N. Donner, R. Bredewold, R. Maloumby, I. Neumann, Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur. J. Neurosci. 25(6), 1804–1814 (2007)
L. Torner, N. Toschi, A. Pohlinger, R. Landgraf, I.D. Neumann, Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J. Neurosci. 21(9), 3207–3214 (2001)
O. Picazo, A. Fernández-Guasti, Changes in experimental anxiety during pregnancy and lactation. Physiol. Behav. 54(2), 295–299 (1993)
D. Toufexis, S. Tesolin, N. Huang, C. Walker, Altered pituitary sensitivity to corticotropin-releasing factor and arginine vasopressin participates in the stress hyporesponsiveness of lactation in the rat. J. Neuroendocrinol. 11(10), 757–764 (1999)
L.D. Sanford, P. Nassar, R.J. Ross, J. Schulkin, A.R. Morrison, Prolactin microinjections into the amygdalar central nucleus lead to decreased NREM sleep. Sleep Res. Online 1, 109–113 (1998)
X. Pi, J.L. Voogt, D.R. Grattan, Detection of prolactin receptor mRNA in the corpus striatum and substantia nigra of the rat. J. Neurosci. Res. 67(4), 551–558 (2002)
B.D. Palma, D.C. Hipolide, S. Tufik, Effects on prolactin secretion and binding to dopaminergic receptors in sleep-deprived lupus-prone mice. Braz. J. Med. Biol. Res. 42(3), 299–304 (2009)
P. Malven, Inhibition of prolactin release in conscious sheep following stimulation of nucleus accumbens and caudate nucleus. Neuroendocrinology 28(3), 160–168 (1979)
R.S.E. Brown, A.E. Herbison, D.R. Grattan, Differential changes in responses of hypothalamic and brainstem neuronal populations to prolactin during lactation in the mouse. Biol. Reprod. 84, 826–836 (2011)
L. Caligaris, S. Taleisnik, Involvement of neurones containing 5-hydroxytryptamine in the mechanism of prolactin release induced by oestrogen. J. Neuroendocrinol. 62(1), 25–33 (1974)
G.A. Jahn, R.P. Deis, Effect of serotonin antagonists on prolactin and progesterone secretion in rats: Evidence that the stimulatory and inhibitory actions of serotonin on prolactin release may be mediated through different receptors. J. Endocrinol. 117(3), 415–422 (1988)
A. Mistry, J.L. Voogt, Role of serotonin in nocturnal and diurnal surges of prolactin in the pregnant rat. Endocrinology 125(6), 2875–2880 (1989)
L. Paut-Pagano, R. Roky, J. Valatx, K. Kitahama, M. Jouvet, Anatomical distribution of prolactin-like immunoreactivity in the rat brain. Neuroendocrinology 58(6), 682–695 (1993)
M. Angoa-Pérez, D. Kuhn, Neuronal serotonin in the regulation of maternal behavior in rodents. Neurotransmitter 2, 615–625 (2015)
M.C. Popesco, A. Frostholm, K. Rejniak, A. Rotter, Digital transcriptome analysis in the aging cerebellum. Ann. N.Y. Acad. Sci. 1019, 58–63 (2004)
A.L. Barofsky, J. Taylor, V.J. Massari, Dorsal raphe-hypothalamic projections provide the stimulatory serotonergic input to suckling-induced prolactin release. Endocrinology 113(5), 1894–1903 (1983)
P. Eriksson, E. Perfilieva, T. Bjork-Eriksson, A. Alborn, C. Nordborg, D. Peterson, F.H. Gage, Neurogenesis in the adult human hippocampus. Nat. Med. 4(11), 1313–1317 (1998)
Acknowledgements
This work was supported by PAPIIT, UNAM grant (IN220315 and IN216817). PhD. Limon-Morales O. is recipient of a DGAPA, UNAM Post-doctoral Fellowship grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Erika Alejandra Cabrera-Reyes and Ofelia Limón-Morales contributed equally to this work
Rights and permissions
About this article
Cite this article
Cabrera-Reyes, E.A., Limón-Morales, O., Rivero-Segura, N.A. et al. Prolactin function and putative expression in the brain. Endocrine 57, 199–213 (2017). https://doi.org/10.1007/s12020-017-1346-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1346-x