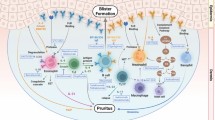
Abstract
Subepidermal autoimmune bullous diseases of the skin and mucosae comprise a large group of chronic diseases, including bullous pemphigoid, pemphigoid gestationis, mucous membrane pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and anti-p200 pemphigoid. These diseases are characterized by an antibody response toward structural components of the basement membrane zone, resulting in subepidermal blistering. The epidemiological features of these diseases vary substantially in different regions of the world. Observational studies investigating comorbidities and associations among patients with these diseases are inconsistent and sometimes inconclusive. This review provides a brief overview regarding each one of the subepidermal autoimmune bullous diseases. In addition, it summarizes the most recent understanding of the epidemiological features and associations of this group of organ-specific autoimmune diseases.
Similar content being viewed by others
References
Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–32.
Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55–70.
Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34
Selmi C, Tsuneyama K. Nutrition, geoepidemiology, and autoimmunity. Autoimmun. Rev. 2010.
Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest. 1988;82:1864–70.
Bernard P, Vaillant L, Labeille B, Bedane C, Arbeille B, Denoeux JP, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch. Dermatol. [internet]. 1995;131:48–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7826096.
Marazza G, Pham HC, Schärer L, Pedrazzetti PP, Hunziker T, Trüeb RM, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br. J. Dermatol. 2009;161:861–8.
Jung M, Kippes W, Messer G, Zillikens D, Rzany B. Increased risk of bullous pemphigoid in male and very old patients: a population-based study on incidence. J. Am. Acad. Dermatol. [Internet]. 1999 [cited 2016 Mar 30];41:266–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10426901.
Della Torre R, Combescure C, Cortés B, Marazza G, Beltraminelli H, Naldi L, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111–7.
Schmidt E, della Torre R, Borradori L. Clinical features and practical diagnosis of bullous pemphigoid. Immunol Allergy Clin North Am. 2012. p. 217–32.
Schmidt E, Sitaru C, Schubert B, Wesselmann U, Kromminga A, Bröcker E-B, et al. Subacute prurigo variant of bullous pemphigoid: autoantibodies show the same specificity compared with classic bullous pemphigoid. J. Am. Acad. Dermatol. [Internet]. 2002 [cited 2016 Sep 30];47:133–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12077594.
Lamb PM, Abell E, Tharp M, Frye R, Deng J-S. Prodromal bullous pemphigoid. Int. J. Dermatol. [Internet]. 2006 [cited 2016 Sep 30];45:209–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16533217.
Strohal R, Rappersberger K, Pehamberger H, Wolff K. Nonbullous pemphigoid: prodrome of bullous pemphigoid or a distinct pemphigoid variant? J. Am. Acad. Dermatol. [Internet]. 1993 [cited 2016 Sep 30];29:293–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8340501.
Levine N, Freilich A, Barland P, Brunsting LA PH, Person JR RRIPH, Barvie A LGBP, et al. Localized pemphigoid simulating dyshidrosiform dermatitis. Arch Dermatol. [Internet]. American Medical Association; 1979 [cited 2016 Sep 30];115:320. Available from: http://archderm.jamanetwork.com/article.aspx?doi=10.1001/archderm.1979.04010030028010
Sawamura D, Li K, Chu ML, Uitto J. Human bullous pemphigoid antigen (BPAG1): amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J Biol Chem. 1991;266:17784–90.
Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J. Clin. Invest. [Internet]. 1993 [cited 2016 Sep 30];92:2480–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7693763.
Stanley JR. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv. Immunol. [Internet]. 1993 [cited 2016 Sep 30];53:291–325. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8512037.
Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. [internet]. J. Invest. Dermatol. 1997. p. 573–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9326393.
Blöcker IM, Dähnrich C, Probst C, Komorowski L, Saschenbrecker S, Schlumberger W, et al. Epitope mapping of BP230 leading to a novel enzyme-linked immunosorbent assay for autoantibodies in bullous pemphigoid. Br J Dermatol. 2012;166:964–70.
Baican A, Baican C, Chiriac G, Chiriac MT, Macovei V, Zillikens D, et al. Pemphigus vulgaris is the most common autoimmune bullous disease in Northwestern Romania. Int J Dermatol. 2010;49:768–74.
Nanda A, Dvorak R, Al-Saeed K, Al-Sabah H, Alsaleh QA. Spectrum of autoimmune bullous diseases in Kuwait. Int J Dermatol. 2004;43:876–81.
Bertram F, Bröcker E-B, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J. Dtsch. Dermatol. Ges. [internet]. 2009;7:434–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19170813.
Joly P, Baricault S, Sparsa A, Bernard P, Bédane C, Duvert-Lehembre S, et al. Incidence and mortality of bullous pemphigoid in France. J. Invest. Dermatol. [internet]. 2012;132:1998–2004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22418872.
Wever S, Wever S, Roth A, Weidenthaler-Barth B, Hashimoto T, Bröcker E-B, et al. Incidence of autoimmune subepidermal blistering dermatoses in a region of Central Germany. Arch. Dermatol. [Internet]. American Medical Association; 1995 [cited 2016 Sep 28];131:957. Available from: http://archderm.jamanetwork.com/article.aspx?doi=10.1001/archderm.1995.01690200097021
Gudi VS, White MI, Cruickshank N, Herriot R, Edwards SL, Nimmot F, et al. Annual incidence and mortality of bullous pemphigoid in the Grampian Region of North-east Scotland. Br J Dermatol. 2005;153:424–7.
Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJP, West J. Bullous pemphigoid and pemphigus vulgaris—incidence and mortality in the UK: population based cohort study. BMJ [Internet]. 2008;337:160–3. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L352039867
Hübner F, Recke A, Zillikens D, Linder R, Schmidt E. Prevalence and age distribution of pemphigus and pemphigoid diseases in Germany. J. Invest. Dermatol. [Internet]. 2016 [cited 2017 Sep 3];136:2495–8. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022202X16321224
Zaraa I, Kerkeni N, Ishak F, Zribi H, El Euch D, Mokni M, et al. Spectrum of autoimmune blistering dermatoses in Tunisia: an 11-year study and a review of the literature. Int. J. Dermatol. [Internet]. 2011 [cited 2016 Feb 17];50:939–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21781064.
Cozzani E, Parodi A, Rebora A, Delmonte S, Barile M, Nigro A, et al. Bullous pemphigoid in Liguria: a 2-year survey. J Eur Acad Dermatology Venereol. 2001;15:317–9.
Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJP, West J. Bullous pemphigoid and pemphigus vulgaris—incidence and mortality in the UK: population based cohort study. BMJ [Internet]. 2008 [cited 2016 Feb 17];337:a180. Available from: http://www.bmj.com/content/337/bmj.a180.full
Uzun S, Durdu M, Akman A, Gunasti S, Uslular C, Memisoglu HR, et al. Pemphigus in the Mediterranean region of Turkey: a study of 148 cases. Int. J. Dermatol. [Internet]. 2006 [cited 2016 Feb 17];45:523–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16700784.
Serwin AB, Bokiniec E, Piascik M, Masny D, Chodynicka B. Epidemiological and clinical analysis of pemphigoid patients in northeastern Poland in 2000-2005. Med Sci Monit Int Med J Exp Clin Res. 2007;13:CR360–4.
Zillikens D, Wever S, Roth A, Weidenthaler-Barth B, Hashimoto T, Bröcker EB. Incidence of autoimmune subepidermal blistering dermatoses in a region of central Germany. Arch. Dermatol. [Internet]. 1995 [cited 2016 Mar 30];131:957–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7669112.
Kridin K, Bergman R. Ethnic variations in the epidemiology of bullous pemphigoid in Israel. Int J Dermatol [Internet]. 2017 Oct 31 [cited 2017 Nov 7]; Available from: http://doi.wiley.com/10.1111/ijd.13813.
Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637–43.
Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: a population-based case-control study. J. Invest. Dermatol. [Internet]. 2011;131:631–6. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emed10&AN=2011087961%5Cn, http://metalib.lib.ic.ac.uk:9003/sfx_local?sid=OVID&isbn=&issn=0022-202X&volume=131&issue=3&date=2011&title=Journal+of+Investigative+Dermatology&atitle=The+relation.
Taghipour K, Chi C-C, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: a case-control study. Arch Dermatol. 2010;146:1251–4. https://doi.org/10.1001/archdermatol.2010.322.
Bastuji-Garin S, Joly P, Picard-Dahan C, Bernard P, Vaillant L, Pauwels C, et al. Drugs associated with bullous pemphigoid. A case-control study. Arch Dermatol. 1996;132:272–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8607630
Delgado JC, Turbay D, Yunis EJ, Yunis JJ, Morton ED, Bhol K, et al. A common major histocompatibility complex class II allele HLA-DQB1* 0301 is present in clinical variants of pemphigoid. Proc. Natl. Acad. Sci. U. S. A. [Internet]. National Academy of Sciences; 1996 [cited 2016 Sep 27];93:8569–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8710911.
Yancey KB. Pemphigoid: clinical, histologic, immunopathologic, and therapeutic considerations. JAMA J. Am. Med. Assoc. [Internet]. 2000;284:350–6. Available from: http://jama.ama-assn.org/cgi/doi/10.1001/jama.284.3.350
Horton R, Wilming L, Rand V, Lovering RC, Bruford EA, Khodiyar VK, et al. Gene map of the extended human MHC. Nat. Rev. Genet. [Internet]. 2004;5:889–99. Available from: http://www.nature.com/doifinder/10.1038/nrg1489
Okazaki A, Miyagawa S, Yamashina Y, Kitamura W, Shirai T. Polymorphisms of HLA-DR and -DQ genes in Japanese patients with bullous pemphigoid. J. Dermatol. [Internet]. 2000 [cited 2016 mar 30];27:149–56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10774139.
Zakka LR, Reche P, Ahmed AR. Role of MHC class II genes in the pathogenesis of pemphigoid. Autoimmun Rev. 2011. p. 40–7.
Ahmed AR, Maize JC, Provost TT. Bullous pemphigoid: clinical and immunologic follow-up after successful therapy. Arch. Dermatol. [Internet]. American Medical Association; 1977 [cited 2017 Apr 1];113:1043. Available from: http://archderm.jamanetwork.com/article.aspx?doi=10.1001/archderm.1977.01640080045002
Roujeau JC, Lok C, Bastuji-Garin S, Mhalla S, Enginger V, Bernard P. High risk of death in elderly patients with extensive bullous pemphigoid. Arch Dermatol [Internet]. 1998;134:465–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9554299.
García-Doval I, Conde Taboada A, Cruces Prado MJ. Sepsis associated with dermatologic hospitalization is not the cause of high mortality of bullous pemphigoid in Europe. J. Invest. Dermatol. [Internet]. 2005 [cited 2017 Mar 24];124:666–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15737212.
Cortes B, Marazza G, Naldi L, Combescure C, Borradori L. Mortality of bullous pemphigoid in Switzerland: a prospective study. Br J Dermatol. 2011;165:368–74.
Gual A, Mascaró JM, Rojas-Farreras S, Guilabert A, Julià M, Iranzo P. Mortality of bullous pemphigoid in the first year after diagnosis: a retrospective study in a Spanish medical centre. J Eur Acad Dermatology Venereol. 2014;28:500–6.
Brick KE, Weaver CH, Lohse CM, Pittelkow MR, Lehman JS, Camilleri MJ, et al. Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J. Am. Acad. Dermatol. [Internet]. 2014 [cited 2017 Mar 24];71:92–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0190962214011475
Cai SCS, Allen JC, Lim YL, Chua SH, Tan SH, Tang MBY. Mortality of bullous pemphigoid in Singapore: risk factors and causes of death in 359 patients seen at the National Skin Centre. Br. J. Dermatol. [Internet]. 2014 [cited 2017 Mar 24];170:1319–26. Available from: http://doi.wiley.com/10.1111/bjd.12806
Lee JH, Kim SC. Mortality of patients with bullous pemphigoid in Korea. J. Am. Acad. Dermatol. [Internet]. 2014 [cited 2017 Mar 24];71:676–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24930586.
Parker SRS, Dyson S, Brisman S, Pennie M, Swerlick RA, Khan R, et al. Mortality of bullous pemphigoid: an evaluation of 223 patients and comparison with the mortality in the general population in the United States. J Am Acad Dermatol. 2008;59:582–8.
Försti A, Jokelainen J, Timonen M, Tasanen K. Risk of death in bullous pemphigoid: a retrospective database study in Finland. Acta Derm. Venereol. [Internet]. 2014 [cited 2017 Mar 29];0. Available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-2347
Phoon YW, Fook-Chong SMC, Koh HY, Thirumoorthy T, Pang SM, Lee HY. Infectious complications in bullous pemphigoid: an analysis of risk factors. J Am Acad Dermatol. 2015;72:834–9.
Rzany B, Partscht K, Jung M, Kippes W, Mecking D, Baima B, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of glucocorticosteroids, and old age. Arch. Dermatol. [internet]. 2002;138:903–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12071817.
Ren Z, Hsu DY, Brieva J, Silverberg NB, Langan SM, Silverberg JI. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87–99.
Joly P, Roujeau J-C, Benichou J, Picard C, Dreno B, Delaporte E, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N. Engl. J. Med. [Internet]. 2002;346:321–7 Available from: http://www.ncbi.nlm.nih.gov/pubmed/11821508.
Pietkiewicz P, Gornowicz-Porowska J, Bowszyc-Dmochowska M, Bartkiewicz P, Dmochowski M. Bullous pemphigoid and neurodegenerative diseases: a study in a setting of a Central European university dermatology department. Aging Clin Exp Res. 2016;28:659–63.
Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC, et al. Comorbidity profiles among patients with bullous pemphigoid: a nationwide population-based study. Br. J. Dermatol. [Internet]. 2011;165:593–9 Available from: http://www.ncbi.nlm.nih.gov/pubmed/21517800.
Gambichler T, Segert H, Hoxtermann S, Schmitz L, Altmeyer P, Teegen B. Neurological disorders in patients with bullous pemphigoid: clinical and experimental investigations. J Eur Acad Dermatol Venereol. 2015;29:1758–62.
Jedlickova H, Hlubinka M, Pavlik T, Semradova V, Budinska E, Vlasin Z. Bullous pemphigoid and internal diseases—a case-control study. Eur J Dermatology. 2010;20:96–101.
Försti A-K, Jokelainen J, Ansakorpi H, Seppänen A, Majamaa K, Timonen M, et al. Psychiatric and neurological disorders are associated with bullous pemphigoid—a nationwide Finnish Care Register study. Sci. Rep. [Internet]. Nature Publishing Group; 2016 [cited 2017 Apr 30];6:37125. Available from: http://www.nature.com/articles/srep37125
Lai Y, Yew Y, Lambert W. Bullous pemphigoid and its association with neurological diseases: a systematic review and meta-analysis. J Eur Acad Dermatology Venereol. 2016;30:2007–15. Available from: http://doi.wiley.com/10.1111/jdv.13660
Brick K, Wieland C, Weaver C, Lohse C, Gibson L, Pittelkow M, et al. A population-based study on the association between bullous pemphigoid and neurologic disease. J. Am. Acad. Dermatol. [Internet]. 2014;70:AB1. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L71390098
Tarazona MJM, Unterstell N, de Macedo Mota ANC, Bressan AL, Gripp AC. Bullous pemphigoid and neurological disease: statistics from a dermatology service. An Bras Dermatol. 2015;90:280–2.
Daneshpazhooh M, Khorassani J, Balighi K, Ghandi N, Mahmoudi H, Tohidinik H, et al. Neurological diseases and bullous pemphigoid: a case-control study in Iranian patients. Indian J Dermatol Venereol Leprol. 2017;83
Chevalier V, Barbe C, Reguiai Z, Plée J, Grange F, Bernard P. Impact pronostique des maladies neurologiques au cours de la pemphigoïde bulleuse : étude rétrospective de 178 cas. Ann. Dermatol. Venereol. [Internet]. 2016 [cited 2017 Sep 10];143:179–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26831943.
Cordel N, Chosidow O, Hellot M-F, Delaporte E, Lok C, Vaillant L, et al. Neurological disorders in patients with bullous pemphigoid. Dermatology [Internet]. 2007;215:187–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17823513.
Amber KT, Zikry J, Hertl M. A multi-hit hypothesis of bullous pemphigoid and associated neurological disease: is HLA-DQB1*03:01, a potential link between immune privileged antigen exposure and epitope spreading? HLA. 2017. p. 127–34.
Li L, Chen J, Wang B, Yao Y, Zuo Y. Sera from patients with bullous pemphigoid (BP) associated with neurological diseases recognized BP antigen 1 in the skin and brain. Br. J. Dermatol. [Internet]. 2009 [cited 2017 Apr 13];160:1343–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19416254.
Brown A, Bernier G, Mathieu M, Rossant J, Kothary R. The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat Genet. 1995;10:301–6.
Seppänen A, Autio-Harmainen H, Alafuzoff I, Särkioja T, Veijola J, Hurskainen T, et al. Collagen XVII is expressed in human CNS neurons. Matrix Biol. 2006;25:185–8.
Claudepierre T, Manglapus MK, Marengi N, Radner S, Champliaud MF, Tasanen K, et al. Collagen XVII and BP AG1 expression in the retina: evidence for an anchoring complex in the central nervous system. J Comp Neurol. 2005;487:190–203.
Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, et al. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–43.
Lindelof B, Islam N, Eklund G, Arfors L. Pemphigoid and cancer. ArchDermatol. 1990;126:66–8.
Ogawa H, Sakuma M, Morioka S, Kitamura K, Sasai Y, Imamura S, et al. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci. 1995;9:136–41.
Schulze F, Neumann K, Recke A, Zillikens D, Linder R, Schmidt E. Malignancies in pemphigus and pemphigoid diseases. J. Invest. Dermatol. [Internet]. 2015 [cited 2017 Apr 14];135:1445–7. Available from: https://ssl.haifa.ac.il/S0022202X15372419/,DanaInfo=.aadBhpxEjlwJn0z+1-s2.0-S0022202X15372419-main.pdf?_tid=48c2b632-211b-11e7-ab18-00000aab0f26&acdnat=1492178852_19abf9717daebe91b688e3cbd8fcbc2e.
Atzmony L, Mimouni I, Reiter O, Leshem YA, Taha O, Gdalevich M, et al. Association of bullous pemphigoid with malignancy: a systematic review and meta-analysis. J Am Acad. Dermatol. 2017;
Muramatsu T, Yamashina Y, Shirai T, Ohnishi T. UVB irradiation reduces the expression of pemphigoid antigens in organ-cultured normal human skin. Arch. Dermatol. Res. [Internet]. 1994 [cited 2016 Dec 29];286:142–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8002665.
Washio H, Hara H, Suzuki H, Yoshida M, Hashimoto T. Bullous pemphigoid on psoriasis lesions after UVA radiation. Acta Derm. Venereol. [Internet]. 2005 [cited 2016 Dec 29];85:561–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16396821.
Ohata C, Ishii N, Koga H, Fukuda S, Tateishi C, Tsuruta D, et al. Coexistence of autoimmune bullous diseases (AIBDs) and psoriasis: a series of 145 cases. J. Am. Acad. Dermatol. [Internet]. 2015 [cited 2016 Dec 29];73:50–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25896671.
Kirtschig G, Chow ET, Venning VA, Wojnarowska FT. Acquired subepidermal bullous diseases associated with psoriasis: a clinical, immunopathological and immunogenetic study. Br J Dermatol [internet]. 1996;135:738–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8977674
Chung SD, Lin HC, Wang KH. Increased risk of pemphigoid following scabies: a population-based matched-cohort study. J. Eur. Acad. Dermatology Venereol. [Internet]. 2014;28:558–64. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84899473507&doi=10.1111%2Fjdv.12132&partnerID=40&md5=1c585d4a38d3411f644043a809bf5f56
Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol. 1999;24:255–9.
Lipozenčić J, Ljubojevic S, Bukvić-Mokos Z. Pemphigoid gestationis. Clin. Dermatol. [Internet]. 2012 [cited 2017 Sep 4];30:51–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22137226
Al-Saif F, Elisa A, Al-Homidy A, Al-Ageel A, Al-Mubarak M. Retrospective analysis of pemphigoid gestationis in 32 Saudi patients—clinicopathological features and a literature review. J Reprod Immunol. 2016;116:42–5.
Tani N, Kimura Y, Koga H, Kawakami T, Ohata C, Ishii N, et al. Clinical and immunological profiles of 25 patients with pemphigoid gestationis. Br J Dermatol. 2015;172:120–9.
Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: clinical features, diagnosis, and management. Clin Rev Allergy Immunol. 2017:1–26.
Ambros-Rudolph CM, Müllegger RR, Vaughan-Jones SA, Kerl H, Black MM, Almuna R, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J. Am. Acad. Dermatol. [Internet]. Mosby, NewYork; 2006 [cited 2017 Sep 4];54:395–404. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0190962205047122
Kolodny RC. Herpes gestationis. A new assessment of incidence, diagnosis, and fetal prognosis. Am. J. Obstet. Gynecol. [Internet]. 1969 [cited 2017 Sep 5];104:39–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5777701.
Shornick JK, Bangert JL, Freeman RG, Gilliam JN. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214–24.
Holmes RC, Black MM, Dann J, James DCO, Bhogal B. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol. 1982;106:499–510.
Zurn A, Celebi CR, Bernard P, Didierjean L, Saurat J-H. A prospective immunofluorescence study of 111 cases of pruritic dermatoses of pregnancy: IgM anti-basement membrane zone antibodies as a novel finding. Br J Dermatol. 1992;126:474–8.
Harrington CI, Bleehen SS. Herpes gestationis: immunopathological and ultrastructural studies. Br. J. Dermatol. [Internet]. 1979 [cited 2017 Sep 5];100:389–99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/378249
Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009. p. 138–44.
Shornick JK, Jenkins RE, Artlett CM, Briggs DC, Welsh KI, Kelly SE, et al. Class II MHC typing in pemphigoid gestationis. Clin. Exp. Dermatol. [Internet]. 1995 [cited 2017 Sep 5];20:123–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8565245
Shornick JK. Herpes gestationis. J Am Acad Dermatol. [Internet]. 1987 [cited 2017 Sep 5];17:539–56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3312312.
Chi C-C, Wang S-H, Charles-Holmes R, Ambros-Rudolph C, Powell J, Jenkins R, et al. Pemphigoid gestationis: early onset and blister formation are associated with adverse pregnancy outcomes. Br J Dermatol. 2009;160:1222–8.
Shornick JK, Black MM. Secondary autoimmune diseases in herpes gestationis (pemphigoid gestationis). J Am Acad Dermatol. 1992;26:563–6.
Holmes RC, Black MM. Herpes gestationis. A possible association with autoimmune thyrotoxicosis (Graves’ disease). J. Am. Acad. Dermatol. [Internet]. 1980 [cited 2017 Sep 7];3:474–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7012197.
Holmes RC, Black MM, Jurecka W, Dann J, James DCO, Timlin D, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol. 1983;109:131–9.
Radford CF, Rauz S, Williams GP, Saw VPJ, Dart JKG. Incidence, presenting features, and diagnosis of cicatrising conjunctivitis in the United Kingdom. Eye [Internet]. 2012;26:1199–208. Available from: http://www.nature.com/doifinder/10.1038/eye.2012.119
Thorne JE, Anhalt GJ, Jabs DA. Mucous membrane pemphigoid and Pseudopemphigoid. Ophthalmology. 2004;111:45–52.
Ahmed AR, Kurgis BS, Rogers III RS. Cicatricial pemphigoid. J Am Acad Dermatol 1991;24.
Alexandre M, Brette MD, Pascal F, Tsianakas P, Fraitag S, Doan S, et al. A prospective study of upper aerodigestive tract manifestations of mucous membrane pemphigoid. Medicine (Baltimore). [Internet]. 2006;85:239–52. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med5&AN=16862049
Higgins GT, Allan RB, Hall R, Field EA, Kaye SB. Development of ocular disease in patients with mucous membrane pemphigoid involving the oral mucosa. Br. J. Ophthalmol. [Internet]. 2006;90:964–7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1857184&tool=pmcentrez&rendertype=abstract.
Laskaris G, Sklavounou A, Stratigos J. Bullous pemphigoid, cicatricial pemphigoid, and pemphigus vulgaris. A comparative clinical survey of 278 cases. Oral Surg Oral Med. Oral Pathol. 1982;54:656–62.
Carrozzo M, Fasano ME, Broccoletti R, Carbone M, Cozzani E, Rendine S, et al. HLA-DQB1 alleles in Italian patients with mucous membrane pemphigoid predominantly affecting the oral cavity. Br. J. Dermatol. [Internet]. 2001 [cited 2017 Sep 3];145:805–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11736906
Chan LS, Hammerberg C, Cooper KD. Significantly increased occurrence of HLA-DQB1*0301 allele in patients with ocular cicatricial pemphigoid. J Invest Dermatol. 1997;108:129–32.
Egan CA, Lazarova Z, Darling TN, Yee C, Cote T, Yancey KB. Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet [Internet]. 2001;357:1850–1. Available from: http://www.sciencedirect.com/science/article/pii/S0140673600049710
Matsushima S, Horiguchi Y, Honda T, Fujii S, Okano T, Tanabe M, et al. A case of anti-epiligrin cicatricial pemphigoid associated with lung carcinoma and severe laryngeal stenosis: review of Japanese cases and evaluation of risk for internal malignancy. J. Dermatol. [Internet]. 2004;31:10–5. Available from: http://search.ebscohost.com/login.aspx?direct=true&db=mdc&AN=14739497&site=ehost-live
Leverkus M, Schmidt E, Lazarova Z, Brocker EB, Yancey KB, Zillikens D. Antiepiligrin cicatricial pemphigoid: an underdiagnosed entity within the spectrum of scarring autoimmune subepidermal bullous diseases? Arch Dermatol [Internet]. 1999;135:1091–8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10490114
Nayar M, Wojnarowska F. No association between cicatricial pemphigoid and malignant disease. Br. J. Dermatol. [Internet]. 1991 [cited 2017 Sep 3];125:193–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1911307
Malik M, Gürcan HM, Christen W, Razzaque AA. Relationship between cancer and oral pemphigoid patients with antibodies to α6-integrin. J Oral Pathol Med. 2007;36:1–5.
Letko E, Gürcan HM, Papaliodis GN, Christen W, Foster CS, Ahmed AR. Relative risk for cancer in mucous membrane pemphigoid associated with antibodies to the beta4 integrin subunit. Clin Exp Dermatol. 2007;32:637–41.
Woodley DT, Briggaman RA, O’Keefe EJ, Inman AO, Queen LL, Gammon WR. Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N Engl J Med [Internet]. 1984;310:1007–13. Available from: http://www.nejm.org/doi/abs/10.1056/NEJM198404193101602
Milinković M V., Janković S, Medenica L, Nikolić M, Reljić V, Popadić S, et al. Incidence of autoimmune bullous diseases in Serbia: a 20-year retrospective study. JDDG J. der Dtsch. Dermatologischen Gesellschaft [Internet]. 2016 [cited 2017 Sep 3];14:995–1005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27767273.
Wong SN, Chua SH. Spectrum of subepidermal immunobullous disorders seen at the National Skin Centre, Singapore: a 2-year review. Br J Dermatol. 2002;147:476–80.
Gammon WR, Heise ER, Burke WA, Fine JD, Woodley DT, Briggaman RA. Increased frequency of HLA-DR2 in patients with autoantibodies to epidermolysis bullosa acquisita antigen: evidence that the expression of autoimmunity to type VII collagen is HLA class II allele associated. J. Invest. Dermatol. [Internet]. 1988 [cited 2017 Sep 3];91:228–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3137269.
Zumelzu C, Le Roux-Villet C, Loiseau P, Busson M, Heller M, Aucouturier F, et al. Black patients of African descent and HLA-DRB1*15:03 frequency overrepresented in epidermolysis bullosa acquisita. J. Invest. Dermatol. [Internet]. 2011;131:2386–93. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022202X15350971
Lee CW, Kim SC, Han H. Distribution of HLA class II alleles in Korean patients with epidermolysis bullosa acquisita. Dermatology. 1996;193:328–9.
Chen M, O’Toole EA, Sanghavi J, Mahmud N, Kelleher D, Eeir D, et al. The epidermolysis bullosa acquisita antigen (type VII collagen) is present in human colon and patients with Crohn’s disease have autoantibodies to type VII collagen. J Invest Dermatol. 2002;118:1059–64.
Kim JH, Kim YH, Kim S-C. Epidermolysis bullosa acquisita: a retrospective clinical analysis of 30 cases. Acta Derm Venereol. 2011;91:307–12.
Hundorfean G, Neurath MF, Sitaru C. Autoimmunity against type VII collagen in inflammatory bowel disease. J Cell Mol Med. 2010;14:2393–403.
Ljubojevic S, Lipozenčić J. Autoimmune bullous diseases associations. Clin. Dermatol. [Internet]. 2012 [cited 2017 Sep 3];30:17–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22137223.
Sitaru C, Mihai S, Otto C, Chiriac MT, Hausser I, Dotterweich B, et al. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest. 2005;115:870–8.
Ishii N, Recke A, Mihai S, Hirose M, Hashimoto T, Zillikens D, et al. Autoantibody-induced intestinal inflammation and weight loss in experimental epidermolysis bullosa acquisita. J Pathol. 2011;224:234–44.
Cardinali C, Caproni M, Bernacchi E, Amato L, Fabbri P. The spectrum of cutaneous manifestations in lupus erythematosus—the Italian experience. Lupus [Internet]. 2000;9:417–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10981645
Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br. J. Dermatol. [Internet]. 1996;135:355–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8949425.
Höfler M The Bradford Hill considerations on causality: a counterfactual perspective. Emerg. Themes Epidemiol. [Internet]. 2005;2:11. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1291382&tool=pmcentrez&rendertype=abstract
Kneisel A, Hertl M. Autoimmune bullous skin diseases. Part 1: clinical manifestations. J Dtsch Dermatol Ges. 2011;9:844–56. quiz 857
Ishiko A, Shimizu H, Masunaga T, Yancey KB, Giudice GJ, Zone JJ, et al. 97 kDa linear IgA bullous dermatosis antigen localizes in the lamina lucida between the NC16A and carboxyl terminal domains of the 180 kDa bullous pemphigoid antigen. J Invest Dermatol. 1998;111:93–6.
Pas HH, Kloosterhuis GJ, Heeres K, van der Meer JB, Jonkman MF. Bullous pemphigoid and linear IgA dermatosis sera recognize a similar 120-kDa keratinocyte collagenous glycoprotein with antigenic cross-reactivity to BP180. J. Invest. Dermatol. [Internet]. 1997;108:423–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9077469.
Wojnarowska F, Marsden RA, Bhogal B, Black MM. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults: a comparative study demonstrating clinical and immunopathologic overlap. J Am Acad Dermatol. 1988;19:792–805.
Adam BA. Bullous diseases in Malaysia: epidemiology and natural history. Int. J. Dermatol. [Internet]. 1992 [cited 2017 Sep 3];31:42–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1737688.
Piamphongsant T, Sirimachan S, Himmunknan P. Juvenile blistering diseases: the problems of diagnosis and treatment. Asian Pacific J. allergy Immunol. [Internet]. 1986 [cited 2017 Sep 3];4:133–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3541947.
Ajithkumar K, Kurian S, Jacob M, Pulimood S. Linear IgA bullous dermatosis in south India. Int J Dermatol. 1997;36:191–3.
Aboobaker J, Wojnarowska FT, Bhogal B, Black MM. Chronic bullous dermatosis of childhood—clinical and immunological features seen in African patients. Clin. Exp. Dermatol. [Internet]. 1991 [cited 2017 Sep 3];16:160–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1934564
Denguezli M, Ben Nejma B, Nouira R, Korbi S, Bardi R, Ayed K, et al. [Iga linear bullous dermatosis in children. A series of 12 Tunisian patients]. Ann. Dermatol. Venereol. [Internet]. 1994 [cited 2017 Sep 3];121:888–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7632006.
Mahé A, Flageul B, Bobin P. [Bullous IgA linear dermatosis of children in Mali]. Ann. Dermatol. Venereol. [Internet]. 1996 [cited 2017 Sep 3];123:544–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9615104
Godfrey K, Wojnarowska F, Leonard J. Linear IgA disease of adults: association with lymphoproliferative malignancy and possible role of other triggering factors. Br. J. Dermatol. [Internet]. 1990;123:447–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2095175
Paige DG, Leonard JN, Wojnarowska F, Fry L. Linear IgA disease and ulcerative colitis. Br. J. Dermatol. [Internet]. 1997;136:779–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9205518.
Wojnarowska F, Bhogal BS, Black MM. Chronic bullous disease of childhood and linear IgA disease of adults are IgA1-mediated diseases. Br J Dermatol. 1994;131:201–4.
Zillikens D, Kawahara Y, Ishiko A, Shimizu H, Mayer J, Rank C V, et al. A novel subepidermal blistering disease with autoantibodies to a 200-kDa antigen of the basement membrane zone. J Invest Dermatol [Internet] 1996;106:1333–8. Available from: http://www.nature.com/jid/journal/v106/n6/abs/5610494a.html%5Cn, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8752680
Chen KR, Shimizu S, Miyakawa S, Ishiko A, Shimizu H, Hashimoto T. Coexistence of psoriasis and an unusual IgG-mediated subepidermal bullous dermatosis: identification of a novel 200-kDa lower lamina lucida target antigen. Br. J. Dermatol. [Internet]. 1996;134:340–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8746353.
Dainichi T, Koga H, Tsuji T, Ishii N, Ohyama B, Ueda A, et al. From anti-p200 pemphigoid to anti-laminin gamma1 pemphigoid. J. Dermatol. 2010. p. 231–8.
Dainichi T, Kurono S, Ohyama B, Ishii N, Sanzen N, Hayashi M, et al. Anti-laminin gamma-1 pemphigoid. Proc. Natl. Acad. Sci. [Internet]. 2009 [cited 2017 Sep 8];106:2800–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19196964
Goletz S, Hashimoto T, Zillikens D, Schmidt E. Anti-p200 pemphigoid. J Am Acad Dermatol. 2014. p. 185–91.
Meijer JM, Diercks GFH, Schmidt E, Pas HH, Jonkman MF. Laboratory diagnosis and clinical profile of anti-p200 pemphigoid. JAMA Dermatology [Internet]. 2016;1–8. Available from: http://archderm.jamanetwork.com/article.aspx?doi=10.1001/jamadermatol.2016.1099.
Commin MH, Schmidt E, Duvert-Lehembre S, Lasek A, Morice C, Estival JL, et al. Clinical and immunological features and outcome of anti-p200 pemphigoid. Br J Dermatol. 2016;175:776–81.
Dilling A, Rose C, Hashimoto T, Zillikens D, Shimanovich I. Anti-p200 pemphigoid: a novel autoimmune subepidermal blistering disease. J Dermatol. 2007;34:1–8.
Serwin AB, Musialkowska E, Piascik M. Incidence and mortality of bullous pemphigoid in north-east Poland (Podlaskie Province), 1999-2012: a retrospective bicentric cohort study. Int. J. Dermatol. [Internet]. 2014 [cited 2017 Mar 29];53:e432–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25041554.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests. No funding was gained for the research.
Rights and permissions
About this article
Cite this article
Kridin, K. Subepidermal autoimmune bullous diseases: overview, epidemiology, and associations. Immunol Res 66, 6–17 (2018). https://doi.org/10.1007/s12026-017-8975-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-017-8975-2