Abstract
Aim
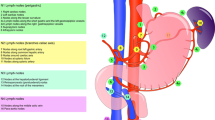
To compare anatomical with numerical criterion to measure the quality of lymphadenectomy for gastric cancer.
Patients and Methods
We analyzed 447 gastric cancer patients with resectable tumor stage (R0 resection) with at least 16 examined lymph nodes.
Results
Of 447 patients, 82.6% underwent D2 lymphadenectomy for a median of total examined lymph nodes of 28. The 7-year disease-specific survival rate for the whole sample was 71.4%. Survival was significantly different between patients treated with D2 and D1 lymphadenectomy (77.4% versus 44.3%; p < 0.001) and between patients with total examined lymph nodes ≥ 28 and < 28 (74.5% versus 62.3%; p = 0.041). Anatomical criterion significantly differentiated 7-year survival in patients stratified according to a numerical parameter.
Conclusion
We should still consider the anatomical criterion as the best item to measure the quality of lymphadenectomy for gastric cancer.

Similar content being viewed by others
References
Qiu MZ, Qiu HJ, Wang ZQ, Ren C, Wang DS, Zhang DS, et al. The tumor - log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLoS One. 2012;7(2):e31736.
Chen HN, Chen XZ, Zhang WH, et al. Necessity of harvesting at least 25 lymph nodes in patients with stage N2–N3 resectable gastric cancer. A 10-year, single-institution cohort study. Medicine. 2015;94(10):e620.
Tanizawa Y, Terashima M. Lymph node dissection in the resection of gastric cancer: review of existing evidence. Gastric Cancer. 2010;13:137–48.
Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al. Nodal dissection for patients with gastric cancer: a randomized controlled trial. Lancet Oncol. 2006;7:309–15.
Wu CW, Hsiung CA, Lo SS. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg. 2004;91:283–7.
Lu J, Wang W, Zheng CH, et al. Influence of total lymph node count on staging and survival after gastrectomy for gastric cancer: an analysis from a two-institution database in China. Ann Surg Oncol. 2017;24:486–93.
Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017; 20(1):1-19.
Giuliani A, Miccini M, Basso L. Extent of lymphadenectomy and perioperative therapies: two open issues in gastric cancer. World J Gastroenterol. 2014;20(14):3889–904.
Deng J, Zhang R, Pan Y, Wang B, Wu L, Hao X, et al. N stages of the seventh edition of TNM classification are the most intensive variables for predictions of the overall survival of gastric cancer patients who underwent limited lymphadenectomy. Tumor Biol. 2014;35(4):3269–81.
Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than number? An analysis of 1.038 patients. Ann Surg. 2000;232(3):362–71.
Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–24.
Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, et al. Patient survival after D1 and D2 resections for gastric cancer: long term results of the MRC randomized surgical trial. Surgical co-operative Group. Br J Cancer. 1999;79:1522–30.
Rausei S, Dionigi G, Sano T, et al. Updates on surgical management of advanced gastric cancer: new evidence and trends. Insights from the first international course on upper gastrointestinal surgery--Varese (Italy), December 2, 2011. Ann Surg Oncol. 2013;20(12):3942–7.
Degiuli M, Sasako M, Ponti A, et al. Morbidity and mortality after D2 gastrectomy for gastric cancer: results of the Italian Gastric Cancer Study Group prospective multicenter surgical study. J Clin Oncol. 1998;16:1490–3.
Barreto SG, Sirohi B. Why should we perform a D2 lymphadenectomy in gastric cancer? Future Oncol. 2017;13(23):2009–12.
Rausei S, Dionigi G, Boni L, Rovera F, Dionigi R. How does the 7th TNM edition fit in gastric cancer management? Ann Surg Oncol. 2011;18(5):1219–21.
Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116(24):5592–8.
Sobin LH, Wittekind CH, editors. TNM classification of malignant tumors. 6th ed. New York: Wiley; 2002.
Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual: TNM classification of malignant tumors. 6th ed. New York: Springer-Verlag; 2002.
In H, Solsky I, Palis B, et al. Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the national cancer database. Ann Surg Oncol. 2017;11:1–9.
Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Gholami S, Janson L, Worhunsky DJ, et al. Number of lymph nodes removed and survival after gastric cancer resection: an analysis from the US gastric cancer collaborative. J Am Coll Surg. 2015;21:291–9.
Schwarz REI, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol. 2006;14:317–8.
Bunt AM, Hermans J, Boon MC, van de Velde C, Sasako M, Fleuren GJ, et al. Evaluation of the extent of lymphadenectomy in a randomized trial of Western-versus Japanese-type surgery in gastric cancer. J Clin Oncol. 1994;12(2):417–22.
Chen T, Yan D, Zheng Z, et al. Evolution in the surgical management of gastric cancer: is extended lymph node dissection back in vogue in the USA? World J Surg Oncol. 2017;15:135.
Chon SH, Berlth F, Plum PS, et al. Gastric cancer treatment in the world: Germany. Transl Gastroenterol Hepatol. 2017;2:53.
Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9(8):775–84.
Nitti D, Marchet A, Olivieri M. Ratio between metastatic and examined lymph node is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitution experience. Ann Surg Oncol. 2003;2(5):S54–7.
Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, et al. The ratio between metastatic and examined lymph nodes is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy. Ann Surg. 2007;245:543–52.
Xu D, Geng Q, Long Z, Zhan YQ, Li W, Zhou ZW, et al. Positive lymph node ratio is an independent prognostic factor in gastric cancer after D2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol. 2009;16:319–26.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• There is no reliable criteria to evaluate the quality of lymphadenectomy for gastric cancer.
• In this multicenter study, we analyzed a large cohort of selected gastric cancer patients to compare anatomical with numerical criterion to measure the quality of the lymphadenectomy.
• Seven-year survival was significantly different between patients treated with D2 and D1 lymphadenectomy and between patients with ≥ 28 and < 28 total examined lymph nodes, but the anatomical criterion was more effective to significantly differentiated patients survival.
• Numerical criterion is too variable to be considered reliable in the evaluating (in a “post hoc” phase) the quality of lymphadenectomy.
Rights and permissions
About this article
Cite this article
Rausei, S., Galli, F., Lianos, G. et al. How Should We Measure the Quality of Lymphadenectomy for Gastric Cancer? Anatomical Versus Numerical Criterion. J Gastrointest Canc 51, 887–892 (2020). https://doi.org/10.1007/s12029-019-00321-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-019-00321-x