Abstract
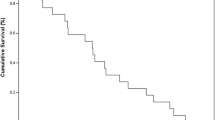
Taxanes and anthracyclines have been among the best-studied chemotherapy classes in castration-resistant prostate cancer (CRPC). Docetaxel (D) 75 mg/m2 every 3 weeks has been the standard first line chemotherapy for CRPC. Encapsulation of doxorubicin in polyethylene glycol-coated liposomes (PLD) was developed to enhance the safety and efficacy of conventional doxorubicin. We hypothesize that the combination of weekly low dose-D and PLD would result in a high response rate and low toxicity. Eligibility criteria included metastatic progressive CRPC, no prior D or PLD and good organ function. After a short phase I with no dose-limiting toxicity, D 30 mg/m2 was administered on days 1, 8 and 15; and PLD 30 mg/m2 on day 1 only, every 28 days. Thirty-seven patients were enrolled. The PSA response rate was 53%. Twenty-two subjects had measurable disease; one (5%) achieved complete response, five (23%) partial response, and twelve (54%) stable disease. Twenty-seven patients (73%) manifested pain relief. The median time to progression was 3.7 months for all patients and 7.9 months for responders. Median overall survival was 16.3 months. Grade 4 neutropenia without infection and anemia occurred in 1 patient each. Grade 3 treatment-related toxicities included: 15% fatigue; 9% neutropenia, anemia and nausea; 6% dehydration and hand-foot syndrome; and 3% infection, febrile neutropenia, thrombosis, stomatitis, headache, vomiting, weight loss and weakness. In this non-comparative study D-PLD demonstrated a higher activity than previously reported with single agent D with favorable side effect profile. A phase 3 study would be needed to evaluate the true benefit of this combination.
ClinicalTrials.gov Identifier: NCT00456989.


Similar content being viewed by others
References
Laber DA, Goetz HK, Bhupalam L. Advances in the management of metastatic androgen-independent prostate cancer. J Ky Med Assoc. 2006;104(2):65–9.
Basch E, Loblaw DA, Oliver TK, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014;32(30):3436–48.
Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–12.
Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20.
Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26(2):242–5.
Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14(6):1756–64.
Vail DM, Amantea MA, Colbern GT, Martin FJ, Hilger RA, Working PK. Pegylated liposomal doxorubicin: proof of principle using preclinical animal models and pharmacokinetic studies. Semin Oncol. 2004;31(6 Suppl 13):16–35.
Harris KA, Harney E, Small EJ. Liposomal doxorubicin for the treatment of hormone-refractory prostate cancer. Clin Prostate Cancer. 2002;1(1):37–41.
Heidenreich A, Sommer F, Ohlmann CH, et al. Prospective randomized Phase II trial of pegylated doxorubicin in the management of symptomatic hormone-refractory prostate carcinoma. Cancer. 2004;101(5):948–56.
Kroon J, Metselaar JM, Storm G, van der Pluijm G. Liposomal nanomedicines in the treatment of prostate cancer. Cancer Treat Rev. 2014;40(4):578–84.
Montanari M, Fabbri F, Rondini E, et al. Phase II trial of non-pegylated liposomal doxorubicin and low-dose prednisone in second-line chemotherapy for hormone-refractory prostate cancer. Tumori. 2012;98(6):696–701.
Laber DA, Glisson SD, Hargis JB, et al. A phase II study of higher dose docetaxel in androgen-independent prostate cancer. Am J Clin Oncol. 2006;29(4):389–94.
de la Fouchardiere C, Largillier R, Goubely Y, et al. Docetaxel and pegylated liposomal doxorubicin combination as first-line therapy for metastatic breast cancer patients: results of the phase II GINECO trial CAPYTTOLE. Ann Oncol. 2009;20(12):1959–63.
Network NCC (2020) Prostate Cancer Version (1.2020). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Published 2020. Accessed 19 Apr 2020
de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54.
Laber DA, Chen MB, Jaglal M, Patel A, Visweshwar N. Phase 2 study of cyclophosphamide, etoposide, and estramustine in patients with castration-resistant prostate cancer. Clin Genitourin Cancer. 2018;16(6):473–81.
Lee JL, Ahn JH, Choi MK, et al. Gemcitabine-oxaliplatin plus prednisolone is active in patients with castration-resistant prostate cancer for whom docetaxel-based chemotherapy failed. Br J Cancer. 2014;110(10):2472–8.
Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19(13):3621–30.
Ladoire S, Eymard JC, Zanetta S, et al. Metronomic oral cyclophosphamide prednisolone chemotherapy is an effective treatment for metastatic hormone-refractory prostate cancer after docetaxel failure. Anticancer Res. 2010;30(10):4317–23.
Loriot Y, Massard C, Gross-Goupil M, et al. Combining carboplatin and etoposide in docetaxel-pretreated patients with castration-resistant prostate cancer: a prospective study evaluating also neuroendocrine features. Ann Oncol. 2009;20(4):703–8.
Nakabayashi M, Sartor O, Jacobus S, et al. Response to docetaxel/carboplatin-based chemotherapy as first- and second-line therapy in patients with metastatic hormone-refractory prostate cancer. BJU Int. 2008;101(3):308–12.
Cabrespine A, Guy L, Khenifar E, et al. Randomized Phase II study comparing paclitaxel and carboplatin versus mitoxantrone in patients with hormone-refractory prostate cancer. Urology. 2006;67(2):354–9.
Beer TM, Garzotto M, Katovic NM. High-dose calcitriol and carboplatin in metastatic androgen-independent prostate cancer. Am J Clin Oncol. 2004;27(5):535–41.
Abratt RP, Brune D, Dimopoulos MA, et al. Randomised phase III study of intravenous vinorelbine plus hormone therapy versus hormone therapy alone in hormone-refractory prostate cancer. Ann Oncol. 2004;15(11):1613–21.
Torti FM, Aston D, Lum BL, et al. Weekly doxorubicin in endocrine-refractory carcinoma of the prostate. J Clin Oncol. 1983;1(8):477–82.
Funding
Grant from Sanofi-Aventis Pharmaceuticals Inc and Ortho Biotech.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose within the past 2 years.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laber, D.A., Eatrides, J., Jaglal, M.V. et al. A phase I/II study of docetaxel in combination with pegylated liposomal doxorubicin in metastatic castration-resistant prostate cancer. Med Oncol 37, 95 (2020). https://doi.org/10.1007/s12032-020-01420-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01420-7