Abstract
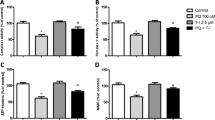
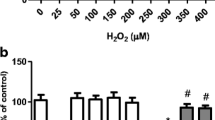
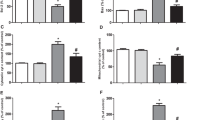
Carnosic acid (CA; C20H28O4), which is also called salvin, is a major phenolic diterpene found in Rosmarinus officinalis L. and exhibits antioxidant, anti-inflammatory, and antiproliferative properties. CA activates the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor, leading to the upregulation of antioxidant and phase II detoxification enzymes, such as heme oxygenase-1 (HO-1), glutathione reductase (GR), γ-glutamate-cysteine ligase (γ-GCL), and glutathione S-transferase (GST), among others. We have previously demonstrated that CA upregulates the total and mitochondrial synthesis of glutathione (GSH), causing mitochondrial protection against paraquat (PQ) and methylglyoxal (MG). Nonetheless, the complete mechanism by which CA prevented mitochondrial dysfunction was not clear yet. Here, we examine whether HO-1 would be involved in the CA-induced mechanism of mitochondrial protection in SH-SY5Y-treated cells. SH-SY5Y cells were pretreated with CA (1 μM) for 12 h prior to a challenge with PQ at 100 μM for additional 24 h. Zinc protoporphyrin IX (ZnPP IX; a specific inhibitor of HO-1; 10 μM) was utilized prior to exposure to CA in order to investigate whether HO-1 was involved in the cytoprotective effects elicited by CA. We found that the CA-induced Nrf2-dependent HO-1 upregulation ameliorated, at least in part, the mitochondrial function in PQ-treated cells. Therefore, CA protected mitochondria of SH-SY5Y cells and exerted anti-apoptotic effects by activating the Nrf2/HO-1 axis.







Similar content being viewed by others
References
Boekema EJ, Braun HP (2007) Supramolecular structure of the mitochondrial oxidative phosphorylation system. J Biol Chem 282:1–4
Papa S, Martino PL, Capitanio G, Gaballo A, De Rasmo D, Signorile A, Petruzzella V (2012) The oxidative phosphorylation system in mammalian mitochondria. Adv Exp Med Biol 942:3–37. doi:10.1007/978-94-007-2869-1_1
Naoi M, Maruyama W, Shamoto-Nagai M, Yi H, Akao Y, Tanaka M (2005) Oxidative stress in mitochondria: decision to survival and death of neurons in neurodegenerative disorders. Mol Neurobiol 31:81–93
Green DR, Galluzzi L, Kroemer G (2014) Metabolic control of cell death. Science 345:1250256. doi:10.1126/science.1250256
Bal-Price A, Brown GC (2000) Nitric-oxide-induced necrosis and apoptosis in PC12 cells mediated by mitochondria. J Neurochem 75:1455–1464
Brown GC, Borutaite V (2001) Nitric oxide, mitochondria, and cell death. IUBMB Life 52:189–195
Brown GC, Borutaite V (2008) Regulation of apoptosis by the redox state of cytochrome c. Biochim Biophys Acta 1777:877–881. doi:10.1016/j.bbabio.2008.03.024
Ryu SY, Peixoto PM, Teijido O, Dejean LM, Kinnally KW (2010) Role of mitochondrial ion channels in cell death. Biofactors 36:255–263. doi:10.1002/biof.101
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1:1269
Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T et al (1989) Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun 163:1450–1455
Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54:823–827
Lestienne P, Nelson I, Riederer P, Reichmann H, Jellinger K (1991) Mitochondrial DNA in postmortem brain from patients with Parkinson’s disease. J Neurochem 56:1819
Calì T, Ottolini D, Brini M (2011) Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors 37:228–240. doi:10.1002/biof.159
Anderson G, Maes M (2014) Neurodegeneration in Parkinson’s disease: interactions of oxidative stress, tryptophan catabolites and depression with mitochondria and sirtuins. Mol Neurobiol 49:771–783. doi:10.1007/s12035-013-8554-z
Solesio ME, Prime TA, Logan A, Murphy MP, Del Mar Arroyo-Jimenez M, Jordán J, Galindo MF (2013) The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson’s disease. Biochim Biophys Acta 1832:174–182. doi:10.1016/j.bbadis.2012.07.009
Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG (2014) Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Biophys Acta 1842:1282–1294. doi:10.1016/j.bbadis.2013.09.007
Whiteman M, Spencer JP, Szeto HH, Armstrong JS (2008) Do mitochondriotropic antioxidants prevent chlorinative stress-induced mitochondrial and cellular injury? Antioxid Redox Signal 10:641–650
Nadtochiy SM, Madukwe J, Hagen F, Brookes PS (2014) Mitochondrially targeted nitro-linoleate: a new tool for the study of cardioprotection. Br J Pharmacol 171:2091–2098. doi:10.1111/bph.12405
Wongrakpanich A, Geary SM, Joiner ML, Anderson ME, Salem AK (2014) Mitochondria-targeting particles. Nanomedicine (Lond) 9:2531–2543. doi:10.2217/nnm.14.161
Pham J, Grundmann O, Elbayoumi T (2015) Mitochondriotropic nanoemulsified genistein-loaded vehicles for cancer therapy. Methods Mol Biol 1265:85–101. doi:10.1007/978-1-4939-2288-8_7
Paliwal R, Rai S, Vaidya B, Mahor S, Gupta PN, Rawat A, Vyas SP (2007) Cell-selective mitochondrial targeting: progress in mitochondrial medicine. Curr Drug Deliv 4:211–224
de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2015b) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv doi. doi:10.1016/j.biotechadv.2015.12.014
Negrette-Guzmán M, García-Niño WR, Tapia E, Zazueta C, Huerta-Yepez S, León-Contreras JC, Hernández-Pando R, Aparicio-Trejo OE et al (2015) Curcumin attenuates gentamicin-induced kidney mitochondrial alterations: possible role of a mitochondrial biogenesis mechanism. Evid Based Complement Alternat Med 2015:917435. doi:10.1155/2015/917435
de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM (2016) Resveratrol and the mitochondria: from triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 1860:727–745. doi:10.1016/j.bbagen.2016.01.017
Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM (2016) Epigallocatechin gallate and mitochondria-a story of life and death. Pharmacol Res 104:70–85. doi:10.1016/j.phrs.2015.12.027
de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM (2015a) The effects of baicalein and baicalin on mitochondrial function and dynamics: a review. Pharmacol Res 100:296–308. doi:10.1016/j.phrs.2015.08.021
de Oliveira MR, Ferreira GC, Schuck PF (2015d) Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: role for PI3K/Akt/Nrf2 pathway. Toxicol in Vitro 32:41–54. doi:10.1016/j.tiv.2015.12.005
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015c) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. doi:10.1016/j.cbi.2015.11.003
Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J et al (2008) Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem 104:1116–1131
Kosaka K, Mimura J, Itoh K, Satoh T, Shimojo Y, Kitajima C, Maruyama A, Yamamoto M et al (2010) Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J Biochem 147:73–81. doi:10.1093/jb/mvp149
Tsai CW, Lin CY, Wang YJ (2011) Carnosic acid induces the NAD(P)H: quinone oxidoreductase 1 expression in rat clone 9 cells through the p38/nuclear factor erythroid-2 related factor 2 pathway. J Nutr 141:2119–2125. doi:10.3945/jn.111.146779
Chen JH, HP O, Lin CY, Lin FJ, CR W, Chang SW, Tsai CW (2012) Carnosic acid prevents 6-hydroxydopamine-induced cell death in SH-SY5Y cells via mediation of glutathione synthesis. Chem Res Toxicol 25:1893–1901. doi:10.1021/tx300171u
Foresti R, Bains SK, Pitchumony TS, de Castro Brás LE, Drago F, Dubois-Randé JL, Bucolo C, Motterlini R (2013) Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol Res 76:132–148. doi:10.1016/j.phrs.2013.07.010
Miller DM, Singh IN, Wang JA, Hall ED (2013) Administration of the Nrf2-ARE activators sulforaphane and carnosic acid attenuates 4-hydroxy-2-nonenal-induced mitochondrial dysfunction ex vivo. Free Radic Biol Med 57:1–9. doi:10.1016/j.freeradbiomed.2012.12.011
de Oliveira MR (2015) The dietary components carnosic acid and carnosol as neuroprotective agents: a mechanistic view. Mol Neurobiol doi. doi:10.1007/s12035-015-9519-1
Lin CY, Wu CR, Chang SW, Wang YJ, Wu JJ, Tsai CW (2015) Induction of the pi class of glutathione S-transferase by carnosic acid in rat clone 9 cells via the p38/Nrf2 pathway. Food Funct 6:1936–1943. doi:10.1039/c4fo01131g
Zhang D, Lee B, Nutter A, Song P, Dolatabadi N, Parker J, Sanz-Blasco S, Newmeyer T et al (2015) Protection from cyanide-induced brain injury by the Nrf2 transcriptional activator carnosic acid. J Neurochem 133:898–908. doi:10.1111/jnc.13074
Miller DM, Singh IN, Wang JA, Hall ED (2015) Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp Neurol 264:103–110. doi:10.1016/j.expneurol.2014.11.008
Pae HO, Kim EC, Chung HT (2008) Integrative survival response evoked by heme oxygenase-1 and heme metabolites. J Clin Biochem Nutr 42:197–203. doi:10.3164/jcbn.2008029
Surh YJ, Kundu JK, Li MH, Na HK, Cha YN (2009) Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch Pharm Res 32:1163–1176. doi:10.1007/s12272-009-1807-8
Catino S, Paciello F, Miceli F, Rolesi R, Troiani D, Calabrese V, Santangelo R, Mancuso C (2016) Ferulic acid regulates the Nrf2/heme oxygenase-1 system and counteracts trimethyltin-induced neuronal damage in the human neuroblastoma cell line SH-SY5Y. Front Pharmacol 6:305. doi:10.3389/fphar.2015.00305
Wang H, Wang Y, Zhao L, Cui Q, Wang Y, Du G (2016) Pinocembrin attenuates MPP(+)-induced neurotoxicity by the induction of heme oxygenase-1 through ERK1/2 pathway. Neurosci Lett 612:104–109. doi:10.1016/j.neulet.2015.11.048
Jiang G, Hu Y, Liu L, Cai J, Peng C, Li Q (2014) Gastrodin protects against MPP(+)-induced oxidative stress by up regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway in human dopaminergic cells. Neurochem Int 75:79–88. doi:10.1016/j.neuint.2014.06.003
Kwon SH, Ma SX, Hwang JY, Lee SY, Jang CG (2015) Involvement of the Nrf2/HO-1 signaling pathway in sulfuretin-induced protection against amyloid beta25-35 neurotoxicity. Neuroscience 304:14–28. doi:10.1016/j.neuroscience.2015.07.030
Hettiarachchi N, Dallas M, Al-Owais M, Griffiths H, Hooper N, Scragg J, Boyle J, Peers C (2014) Heme oxygenase-1 protects against Alzheimer’s amyloid-β(1-42)-induced toxicity via carbon monoxide production. Cell Death Dis 5:e1569. doi: 10.1038/cddis.2014.529
Lin TK, Chen SD, Chuang YC, Lin HY, Huang CR, Chuang JH, Wang PW, Huang ST et al (2014) Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int J Mol Sci 15:1625–1646. doi:10.3390/ijms15011625
Gill AJ, Kovacsics CE, Cross SA, Vance PJ, Kolson LL, Jordan-Sciutto KL, Gelman BB, Kolson DL (2014) Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J Clin Invest 124:4459–4472. doi:10.1172/JCI72279
Radhakrishnan N, Yadav SP, Sachdeva A, Pruthi PK, Sawhney S, Piplani T, Wada T, Yachie A (2011) Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol 33:74–78. doi:10.1097/MPH.0b013e3181fd2aae
True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG et al (2007) Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res 101:893–901
Negi G, Nakkina V, Kamble P, Sharma SS (2015) Heme oxygenase-1, a novel target for the treatment of diabetic complications: focus on diabetic peripheral neuropathy. Pharmacol Res 102:158–167. doi:10.1016/j.phrs.2015.09.014
Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, Tsatsakis AM, Duarte JA, Carvalho F (2014) Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases--a mechanistic approach. Toxicol Lett 230:85–103. doi:10.1016/j.toxlet.2014.01.039
Huang CL, Chao CC, Lee YC, Lu MK, Cheng JJ, Yang YC, Wang VC, Chang WC et al (2015) Paraquat induces cell death through impairing mitochondrial membrane permeability. Mol Neurobiol doi. doi:10.1007/s12035-015-9198-y
Moran JM, Gonzalez-Polo RA, Ortiz-Ortiz MA, Niso-Santano M, Soler G, Fuentes JM (2008) Identification of genes associated with paraquat-induced toxicity in SH-SY5Y cells by PCR array focused on apoptotic pathways. J Toxicol Environ Health A 71:1457–1467. doi:10.1080/15287390802329364
Ortiz-Ortiz MA, Morán JM, González-Polo RA, Niso-Santano M, Soler G, Bravo-San Pedro JM, Fuentes JM (2009) Nitric oxide-mediated toxicity in paraquat-exposed SH-SY5Y cells: a protective role of 7-nitroindazole. Neurotox Res 16:160–173. doi:10.1007/s12640-009-9065-6
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328:85–92
de Oliveira MR, Moreira JC (2007) Acute and chronic vitamin a supplementation at therapeutic doses induces oxidative stress in submitochondrial particles isolated from cerebral cortex and cerebellum of adult rats. Toxicol Lett 173:145–150
Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L et al (2014) Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 34:227–234. doi:10.1007/s10571-013-0006-9
de Oliveira MR, Lorenzi R, Schnorr CE, Morrone M, Moreira JC (2011) Increased 3-nitrotyrosine levels in mitochondrial membranes and impaired respiratory chain activity in brain regions of adult female rats submitted to daily vitamin a supplementation for 2 months. Brain Res Bull 86:246–253. doi:10.1016/j.brainresbull.2011.08.006
de Oliveira MR, da Rocha RF, Schnorr CE, Moreira JC (2012) L-NAME cotreatment did prevent neither mitochondrial impairment nor behavioral abnormalities in adult Wistar rats treated with vitamin a supplementation. Fundam Clin Pharmacol 26:513–529. doi:10.1111/j.1472-8206.2011.00943.x
de Oliveira MR, Oliveira MW, Da Rocha RF, Moreira JC (2009) Vitamin a supplementation at pharmacological doses induces nitrosative stress on the hypothalamus of adult Wistar rats. Chem Biol Interact 180:407–413. doi:10.1016/j.cbi.2009.02.006
Sun J, Ren X, Simpkins JW (2015) Sequential upregulation of superoxide dismutase 2 and heme oxygenase 1 by tert-Butylhydroquinone protects mitochondria during oxidative stress. Mol Pharmacol 88:437–449. doi:10.1124/mol.115.098269
Yang Y, Wang J, Li Y, Fan C, Jiang S, Zhao L, Di S, Xin Z et al (2015) HO-1 signaling activation by pterostilbene treatment attenuates mitochondrial oxidative damage induced by cerebral ischemia reperfusion injury. Mol Neurobiol doi. doi:10.1007/s12035-015-9194-2
Hwang JH, Kim YH, Noh JR, Gang GT, Kim KS, Chung HK, Tadi S, Yim YH et al (2015) The protective role of NAD(P)H:quinone oxidoreductase 1 on acetaminophen-induced liver injury is associated with prevention of adenosine triphosphate depletion and improvement of mitochondrial dysfunction. Arch Toxicol 89:2159–2166. doi:10.1007/s00204-014-1340-5
Kwon J, Han E, Bui CB, Shin W, Lee J, Lee S, Choi YB, Lee AH et al (2012) Assurance of mitochondrial integrity and mammalian longevity by the p62-Keap1-Nrf2-Nqo1 cascade. EMBO Rep 13:150–156. doi:10.1038/embor.2011.246
Acknowledgments
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). PFS and CSG receive a “CNPq Produtividade em Pesquisa” fellow. GCF is supported by Edital APQ1/FAPERJ and by CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., Peres, A., Ferreira, G.C. et al. Carnosic Acid Protects Mitochondria of Human Neuroblastoma SH-SY5Y Cells Exposed to Paraquat Through Activation of the Nrf2/HO-1Axis. Mol Neurobiol 54, 5961–5972 (2017). https://doi.org/10.1007/s12035-016-0100-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0100-3