Abstract
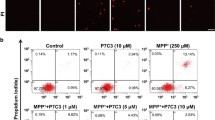
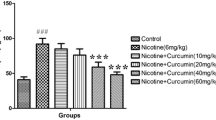
Earlier, protective role of curcumin in arsenic-induced dopamine (DA)–D2 receptor dysfunctions in corpus striatum has been demonstrated by us. In continuation to that, the present study is focused to decipher the molecular mechanisms associated with alterations in dopaminergic signaling on arsenic exposure in corpus striatum and assess the protective efficacy of curcumin. Exposure to arsenic (20 mg/kg, body weight p.o. for 28 days) in rats resulted to decrease the expression of presynaptic proteins-tyrosine hydroxylase and VMAT2 while no effect was observed on the expression of DAT in comparison to controls. A significant decrease in the expression of DA-D2 receptors associated with alterations in the expression of PKA, pDARPP32 (Thr 34), and PP1 α was clearly evident on arsenic exposure. Expression of BDNF and pGSK3β in corpus striatum was found decreased in arsenic-exposed rats. Simultaneous treatment with curcumin (100 mg/kg, body weight p.o. for 28 days) resulted to protect arsenic-induced alterations in the expression of DA-D2 receptors, PKA, pDARPP32, pCREB, and pPP1α. Neuroprotective efficacy of curcumin can possibly be attributed to its antioxidant potential which significantly protected arsenic-induced mitochondrial dysfunctions by modulating the ROS generation and apoptosis. Modulation in the expression of BDNF and pGSK3β in corpus striatum by curcumin exhibits the importance of neuronal survival pathway in arsenic-induced dopaminergic dysfunctions. Interestingly, curcumin was also found to protect arsenic-induced ultrastructural changes in corpus striatum. The results exhibit that curcumin modulates BDNF/DARPP32/CREB in arsenic-induced alterations in dopaminergic signaling in rat corpus striatum.











Similar content being viewed by others
References
Fujihara J, Yasuda T, Kato H, Yuasa I, Panduro A, Kunito T, Takeshita H (2011) Genetic variants associated with arsenic metabolism within human arsenic (+ 3 oxidation state) methyltransferase show wide variation across multiple populations. Arch Toxicol 85(2):119–125
Joseph T, Dubey B, McBean EA (2015) A critical review of arsenic exposures for Bangladeshi adults. Sci Total Environ 527:540–551
Matschullat J (2000) Arsenic in the geosphere—a review. Sci Total Environ 249(1):297–312
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133(1):1–16
Mukherjee A, Sengupta MK, Hossain MA, Ahamed S, Das B, Nayak B, Lodh D, Rahman MM, et al. (2006) Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario. J Health Popul Nutr 142–163
Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environmental Health Perspectives (Online) 121(3):295
Meharg AA, Rahman MM (2003) Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environmental Science & Technology 37(2):229–234
Efferth T, Li PC, Konkimalla VSB, Kaina B (2007) From traditional Chinese medicine to rational cancer therapy. Trends Mol Med 13(8):353–361
Smith C, Livingston S, Doolittle D (1997) An international literature survey of “IARC Group I carcinogens” reported in mainstream cigarette smoke. Food Chem Toxicol 35(10):1107–1130
ATSDR U (2007) Toxicological profile for arsenic. Agency for Toxic Substances and Disease Registry. Division of Toxicology, Atlanta, GA
Tsuji JS, Perez V, Garry MR, Alexander DD (2014) Association of low-level arsenic exposure in drinking water with cardiovascular disease: a systematic review and risk assessment. Toxicology 323:78–94
Wang S-L, Chang F-H, Liou S-H, Wang H-J, Li W-F, Hsieh DP (2007) Inorganic arsenic exposure and its relation to metabolic syndrome in an industrial area of Taiwan. Environ Int 33(6):805–811
Fan Y, Jiang Y, Hu P, Chang R, Yao S, Wang B, Li X, Zhou Q, et al. (2016) Modification of association between prior lung disease and lung cancer by inhaled arsenic: a prospective occupational-based cohort study in Yunnan, China. J Expo Sci Environ Epidemiol
Ferrario D, Gribaldo L, Hartung T (2016) Arsenic exposure and immunotoxicity: a review including the possible influence of age and sex. Current environmental health reports 3(1):1–12
Vibol S, Hashim JH, Sarmani S (2015) Neurobehavioral effects of arsenic exposure among secondary school children in the Kandal Province, Cambodia. Environ Res 137:329–337
Hamadani J, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, Arifeen S, Huda SN et al (2011) Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol 40(6):1593–1604
Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, Garcia-Vargas G, del Carmen Caamaño M, et al. (2007) Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ Health Perspect 1371–1375
Tsai S-Y, Chou H-Y, The H-W, Chen C-M, Chen C-J (2003) The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 24(4):747–753
Mukherjee B, Bindhani B, Saha H, Sinha D, Ray MR (2014) Platelet hyperactivity, neurobehavioral symptoms and depression among Indian women chronically exposed to low level of arsenic. Neurotoxicology 45:159–167
Tyler CR, Allan AM (2014) The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Current environmental health reports 1(2):132–147
Chandravanshi LP, Yadav RS, Shukla RK, Singh A, Sultana S, Pant AB, Parmar D, Khanna VK (2014) Reversibility of changes in brain cholinergic receptors and acetylcholinesterase activity in rats following early life arsenic exposure. Int J Dev Neurosci 34:60–75
Yadav RS, Chandravanshi LP, Shukla RK, Sankhwar ML, Ansari RW, Shukla PK, Pant AB, Khanna VK (2011) Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. Neurotoxicology 32(6):760–768
Yadav RS, Sankhwar ML, Shukla RK, Chandra R, Pant AB, Islam F, Khanna VK (2009) Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol Appl Pharmacol 240(3):367–376
Srivastava P, Yadav RS, Chandravanshi LP, Shukla RK, Dhuriya YK, Chauhan LK, Dwivedi HN, Pant AB et al (2014) Unraveling the mechanism of neuroprotection of curcumin in arsenic induced cholinergic dysfunctions in rats. Toxicol Appl Pharmacol 279(3):428–440
Bardullas U, Limón-Pacheco J, Giordano M, Carrizales L, Mendoza-Trejo M, Rodríguez V (2009) Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol 239(2):169–177
Kim M, Seo S, Sung K, Kim K (2014) Arsenic exposure in drinking water alters the dopamine system in the brains of C57BL/6 mice. Biol Trace Elem Res 162(1–3):175–180
Yadav RS, Shukla RK, Sankhwar ML, Patel DK, Ansari RW, Pant AB, Islam F, Khanna VK (2010) Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in rats. Neurotoxicology 31(5):533–539
Epstein J, Sanderson IR, MacDonald TT (2010) Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 103(11):1545–1557
Jagatha B, Mythri RB, Vali S, Bharath MS (2008) Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radic Biol Med 44(5):907–917
Tripanichkul W, Jaroensuppaperch E (2013) Ameliorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice. Eur Rev Med Pharmacol Sci 17(10):1360–1368
Kulkarni S, Dhir A (2010) An overview of curcumin in neurological disorders. Indian journal of pharmaceutical sciences 72(2):149
Yu J-J, Pei L-B, Zhang Y, Wen Z-Y, Yang J-L (2015) Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. J Clin Psychopharmacol 35(4):406–410
Lopresti AL, Maes M, Meddens MJ, Maker GL, Arnoldussen E, Drummond PD (2015) Curcumin and major depression: a randomised, double-blind, placebo-controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur Neuropsychopharmacol 25(1):38–50
Al-Karawi D, Mamoori A, Alem D, Tayyar Y (2016) The role of curcumin administration in patients with major depressive disorder: mini meta-analysis of clinical trials. Phytother Res 30(2):175–183
Glowinski J, Iversen L (1966) Regional studies of catecholamines in rat brain. 3. Subcellullar distribution of endogenous and exogenous catecholamines in various brain regions. Biochem Pharmacol 15(7):977–97&
Bagh MB, Maiti AK, Jana S, Banerjee K, Roy A, Chakrabarti S (2008) Quinone and oxyradical scavenging properties of N-acetylcysteine prevent dopamine mediated inhibition of Na+, K+-ATPase and mitochondrial electron transport chain activity in rat brain: implications in the neuroprotective therapy of Parkinson’s disease. Free Radic Res 42(6):574–581
Socci D, Bjugstad K, Jones H, Pattisapu J, Arendash G (1999) Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol 155(1):109–117
Kapoor N, Pant AB, Dhawan A, Dwievedi UN, Gupta YK, Seth PK, Parmar D (2006) Differences in sensitivity of cultured rat brain neuronal and glial cytochrome P450 2E1 to ethanol. Life Sci 79(16):1514–1522
Hatefi Y (1978) [1] Introduction—preparation and properties of the enzymes and enzyme complexes of the mitochondrial oxidative phosphorylation system. Methods Enzymol 53:3–4
Clark J, Bates T, Boakye P, Kuimov A, Land J (1997) Investigation of mitochondrial defects in brain and skeletal muscle. In: Neurochemistry: a practical approach. Oxford University Press Oxford, pp 151–174
Wharton DC, Tzagoloff A (1967) [45] Cytochrome oxidase from beef heart mitochondria. Methods Enzymol 10:245–250
Ulmer VerlagLowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265275
Beaulieu J-M, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63(1):182–217
Jones DC, Miller GW (2008) The effects of environmental neurotoxicants on the dopaminergic system: a possible role in drug addiction. Biochem Pharmacol 76(5):569–581
De Mei C, Ramos M, Iitaka C, Borrelli E (2009) Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol 9(1):53–58
Zhang M, Creese I (1993) Antisense oligodeoxynucleotide reduces brain dopamine D2 receptors: behavioral correlates. Neurosci Lett 161(2):223–226
Rodríguez VM, Limón-Pacheco JH, Carrizales L, Mendoza-Trejo MS, Giordano M (2010) Chronic exposure to low levels of inorganic arsenic causes alterations in locomotor activity and in the expression of dopaminergic and antioxidant systems in the albino rat. Neurotoxicol Teratol 32(6):640–647
Listos J, Baranowska-Bosiacka I, Talarek S, Listos P, Orzelska J, Fidecka S, Gutowska I, Kolasa A et al (2013) The effect of perinatal lead exposure on dopamine receptor D2 expression in morphine dependent rats. Toxicology 310:73–83
Greengard P, Allen PB, Nairn AC (1999) Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23(3):435–447
Cordova FM, Aguiar AS Jr, Peres TV, Lopes MW, Gonçalves FM, Remor AP, Lopes SC, Pilati C et al (2012) In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function. PLoS One 7(3):e33057
Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm BB, Fisone G (2000) Regulation of the phosphorylation of the dopamine-and cAMP-regulated phosphoprotein of 32 kDa in vivo by dopamine D1, dopamine D2, and adenosine A2A receptors. Proc Natl Acad Sci 97(4):1856–1860
Rodrıguez V, Carrizales L, Jimenez-Capdeville M, Dufour L, Giordano M (2001) The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull 55(2):301–308
Tadanobu I, Zhang YF, Shigeo M, Hiroko S, Hiromichi N, Hiroki M, Yuichi S, Eiichi A (1990) The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol Lett 54(2–3):345–353
Delgado J, Dufour L, Grimaldo J, Carrizales L, Rodrıguez V, Jimenez-Capdeville M (2000) Effects of arsenite on central monoamines and plasmatic levels of adrenocorticotropic hormone (ACTH) in mice. Toxicol Lett 117(1):61–67
Vahidnia A, van der Straaten R, Romijn F, Van Pelt J, van der Voet G, de Wolff F (2007) Arsenic metabolites affect expression of the neurofilament and tau genes: an in-vitro study into the mechanism of arsenic neurotoxicity. Toxicol in Vitro 21(6):1104–1112
Li X, Zhang H, Niu Q, Yuan F (2012) Changes of cdk5, p35 and p53 gene expression levels in arsenic-induced neural cell apoptosis. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi= Chinese journal of industrial hygiene and occupational diseases 30(2):85–88
Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK et al (2001) Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410(6826):376–380
Sakamoto K, Karelina K, Obrietan K (2011) CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem 116(1):1–9
Sun B-F, Wang Q-Q, Yu Z-J, Yu Y, Xiao C-L, Kang C-S, Ge G, Linghu Y et al (2015) Exercise prevents memory impairment induced by arsenic exposure in mice: implication of hippocampal BDNF and CREB. PLoS One 10(9):e0137810
Sriram K, Lin GX, Jefferson AM, Roberts JR, Chapman RS, Chen BT, Soukup JM, Ghio AJ et al (2010) Dopaminergic neurotoxicity following pulmonary exposure to manganese-containing welding fumes. Arch Toxicol 84(7):521–540
Choi W-S, Kim H-W, Xia Z (2015) JNK inhibition of VMAT2 contributes to rotenone-induced oxidative stress and dopamine neuron death. Toxicology 328:75–81
Ho PW, Ho JW, Liu H-F, So DH, Zero H, Chan K-H, Ramsden DB, Ho S-L (2012) Mitochondrial neuronal uncoupling proteins: a target for potential disease-modification in Parkinson's disease. Translational neurodegeneration 1(1):1
Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ (2010) Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468(7324):696–700
Dwivedi N, Mehta A, Yadav A, Binukumar B, Gill KD, Flora SJ (2011) MiADMSA reverses impaired mitochondrial energy metabolism and neuronal apoptotic cell death after arsenic exposure in rats. Toxicol Appl Pharmacol 256(3):241–248
Baquet ZC, Gorski JA, Jones KR (2004) Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci 24(17):4250–4258
Kumar TP, Antony S, Gireesh G, George N, Paulose C (2010) Curcumin modulates dopaminergic receptor, CREB and phospholipase C gene expression in the cerebral cortex and cerebellum of streptozotocin induced diabetic rats. J Biomed Sci 17(1):1
Hickey MA, Zhu C, Medvedeva V, Lerner RP, Patassini S, Franich NR, Maiti P, Frautschy SA et al (2012) Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol Neurodegener 7(1):1
Du X-X, Xu H-M, Jiang H, Song N, Wang J, Xie J-X (2012) Curcumin protects nigral dopaminergic neurons by iron-chelation in the 6-hydroxydopamine rat model of Parkinson’s disease. Neurosci Bull 28(3):253–258
Jiang T, Wang L, Zhang S, Sun P-C, Ding C-F, Chu Y-Q, Zhou P (2011) Interaction of curcumin with Al (III) and its complex structures based on experiments and theoretical calculations. J Mol Struct 1004(1):163–173
Jiang T, Zhi X-L, Zhang Y-H, Pan L-F, Zhou P (2012) Inhibitory effect of curcumin on the Al (III)-induced Aβ 42 aggregation and neurotoxicity in vitro. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1822(8):1207–1215
Khatri DK, Juvekar AR (2016) Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson’s disease. Pharmacol Biochem Behav 150:39–47
Kunwar A, Priyadarsini KI (2016) Curcumin and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB (eds) Anti-inflammatory nutraceuticals and chronic diseases. Springer International Publishing, Cham, pp. 1–25. doi:10.1007/978-3-319-41334-1_1
Goozee K, Shah T, Sohrabi HR, Rainey-Smith S, Brown B, Verdile G, Martins R (2016) Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr 115(03):449–465
Dairam A, Limson JL, Watkins GM, Antunes E, Daya S (2007) Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J Agric Food Chem 55(3):1039–1044
Lv H, Liu J, Wang L, Zhang H, Yu S, Li Z, Jiang F, Niu Y et al (2014) Ameliorating effects of combined curcumin and desferrioxamine on 6-OHDA-induced rat mode of Parkinson’s disease. Cell Biochem Biophys 70(2):1433–1438
Gao S, Duan X, Wang X, Dong D, Liu D, Li X, Sun G, Li B (2013) Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem Toxicol 59:739–747
Nicolson GL (2013) Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Altern Ther Health Med 20(1):18–25
Pan J, Li H, Ma J-F, Tan Y-Y, Xiao Q, Ding J-Q, Chen S-D (2012) Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Translational neurodegeneration 1(1):1
Wu J, Li Q, Wang X, Yu S, Li L, Wu X, Chen Y, Zhao J et al (2013) Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One 8(3):e59843
Yang C, Zhang X, Fan H, Liu Y (2009) Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res 1282:133–141
Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT (2005) Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res 39(10):1119–1125
Yu S, Zheng W, Xin N, Chi Z-H, Wang N-Q, Nie Y-X, Feng W-Y, Wang Z-Y (2010) Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenation Res 13(1):55–64
Franco-Robles E, Campos-Cervantes A, Murillo-Ortiz BO, Segovia J, López-Briones S, Vergara P, Pérez-Vázquez V, Solís-Ortiz MS et al (2013) Effects of curcumin on brain-derived neurotrophic factor levels and oxidative damage in obesity and diabetes. Appl Physiol Nutr Metab 39(2):211–218
Baydyuk M, Xu B (2014) BDNF signaling and survival of striatal neurons. Front Cell Neurosci 8:254
Wang R, Li Y-H, Xu Y, Li Y-B, Wu H-L, Guo H, Zhang J-Z, Zhang J-J et al (2010) Curcumin produces neuroprotective effects via activating brain-derived neurotrophic factor/TrkB-dependent MAPK and PI-3K cascades in rodent cortical neurons. Prog Neuro-Psychopharmacol Biol Psychiatry 34(1):147–153
Acknowledgements
The study has been carried out as a part of INDEPTH programme of Council of Scientific and Industrial Research (CSIR), New Delhi. The authors thank Director, CSIR-Indian Institute of Toxicology Research (CSIR-IITR), Lucknow, for his support throughout the study and Dr. LKS Chauhan, for the help to carry out experiments involving transmission electron microscopy. Pranay Srivastava and Richa Gupta are grateful to the Council for Scientific and Industrial Research, New Delhi, for the award of Senior Research Fellowship. Technical support by Mr. B.S. Pandey is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were carried out in sync with the guidelines laid down by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests (Government of India), New Delhi, India, and the experimental procedures were approved by the Institutional Animal Ethics Committee of CSIR-IITR, Lucknow (ITRC/IAEC/39/12).
Additional information
Highlights
• The role of DARPP32 in the neuroprotective efficacy of curcumin in arsenic induced dopaminergic alterations.
• BDNF plays a vital role in survival of striatal neurons on arsenic exposure and is involved in neuroprotective role of curcumin.
• Modulation of mitochondrial functions by curcumin to exert antioxidant effect seems to be a pertinent mechanism through which curcumin exercises its effects.
Pranay Srivastava and Yogesh K. Dhuriya contributed equally.
Rights and permissions
About this article
Cite this article
Srivastava, P., Dhuriya, Y.K., Gupta, R. et al. Protective Effect of Curcumin by Modulating BDNF/DARPP32/CREB in Arsenic-Induced Alterations in Dopaminergic Signaling in Rat Corpus Striatum. Mol Neurobiol 55, 445–461 (2018). https://doi.org/10.1007/s12035-016-0288-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0288-2