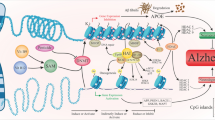
Abstract
Research over the years has shown that causes of Alzheimer’s disease are not well understood, but over the past years, the involvement of epigenetic mechanisms in the developing memory formation either under pathological or physiological conditions has become clear. The term epigenetics represents the heredity of changes in phenotype that are independent of altered DNA sequences. Different studies validated that cytosine methylation of genomic DNA decreases with age in different tissues of mammals, and therefore, the role of epigenetic factors in developing neurological disorders in aging has been under focus. In this review, we summarized and reviewed the involvement of different epigenetic mechanisms especially the DNA methylation in Alzheimer’s disease (AD), late-onset Alzheimer’s disease (LOAD), familial Alzheimer’s disease (FAD), and autosomal dominant Alzheimer’s disease (ADAD). Down to the minutest of details, we tried to discuss the methylation patterns like mitochondrial DNA methylation and ribosomal DNA (rDNA) methylation. Additionally, we mentioned some therapeutic approaches related to epigenetics, which could provide a potential cure for AD. Moreover, we reviewed some recent studies that validate DNA methylation as a potential biomarker and its role in AD. We hope that this review will provide new insights into the understanding of AD pathogenesis from the epigenetic perspective especially from the perspective of DNA methylation.

Similar content being viewed by others
References
Burns A, Iliffe S (2009) Alzheimer’s disease. BMJ 338:b158–b158. doi:10.1136/bmj.b158
World Health Organization (2012) Dementia fact sheet no. 362. http://www.who.int/mediacentre/factsheets/fs362/en/. Accessed 8 Jun 2016
National Institute on Aging (2011) About Alzheimer’s disease: symptoms. http://www.nia.nih.gov/alzheimers/topics/symptoms. Accessed 8 Jun 2016
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362:329–344. doi:10.1056/NEJMra0909142
Todd S, Barr S, Roberts M, Passmore AP (2013) Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry 28:1109–1124. doi:10.1002/gps.3946
Ballard C, Gauthier S, Corbett A et al (2011) Alzheimer’s disease. Lancet 377:1019–1031. doi:10.1016/S0140-6736(10)61349-9
National Institute for Health and Care Excellence (NICE) (2016) Dementia diagnosis and assessment. National Institute for Health and Care Excellence (NICE)
National Institute on Aging (2006) More research needed on ways to prevent Alzheimer’s, panel finds. In: Natl. Inst. Aging. https://www.nia.nih.gov/alzheimers/announcements/2010/06/more-research-needed-ways-prevent-alzheimers-panel-finds
Holliday R (1994) Epigenetics: an overview. Dev Genet 15:453–457. doi:10.1002/dvg.1020150602
Day JJ, Sweatt JD (2011) Epigenetic mechanisms in cognition. Neuron 70:813–829. doi:10.1016/j.neuron.2011.05.019
Fischer A, Sananbenesi F, Mungenast A, Tsai L-H (2010) Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci 31:605–617. doi:10.1016/j.tips.2010.09.003
Sananbenesi F, Fischer A (2009) The epigenetic bottleneck of neurodegenerative and psychiatric diseases. Biol Chem. doi:10.1515/BC.2009.131
Stilling RM, Fischer A (2011) The role of histone acetylation in age-associated memory impairment and Alzheimer’s disease. Neurobiol Learn Mem 96:19–26. doi:10.1016/j.nlm.2011.04.002
Razin A, Riggs AD (1980) DNA methylation and gene function. Science 210:604–610. doi:10.1126/science.6254144
van Emburgh BO, Robertson KD (2008) DNA methyltransferases and methyl-CpG binding proteins as multifunctional regulators of chromatin structure and development in mammalian cells. In: Tost J (ed) Epigenetics. Caister Academic Press, Norfolk, pp. 22–61
Schaefer M, Pollex T, Hanna K et al (2010) RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev 24:1590–1595. doi:10.1101/gad.586710
Jalili M, Pati S, Rath B, et al. (2013) Effect of diet and nutrients on molecular mechanism of gene expression mediated by nuclear receptor and epigenetic modulation. Open Nutraceuticals J 27–34. doi:10.2174/1876396001306010027
Okano M, Bell DW, Haber DA, Li E (1999) DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99:247–257. doi:10.1016/S0092-8674(00)81656-6
Wu H, Tao J, Sun YE (2012) Regulation and function of mammalian DNA methylation patterns: a genomic perspective. 11:240–250. doi:10.1093/bfgp/els011
Suzuki MM, Bird A (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 9:465–476. doi:10.1038/nrg2341
Kriaucionis S, Heintz N (2009) The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science (80- ) 324:929–930. doi:10.1126/science.1169786
Tahiliani M, Koh KP, Shen Y et al (2009) Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324:930–935. doi:10.1126/science.1170116
Xu GL, Wong J (2015) Oxidative DNA demethylation mediated by Tet enzymes. Natl Sci Rev 2:318–328. doi:10.1093/nsr/nwv029
Tan L, Shi YG (2012) Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development 139:1895–1902. doi:10.1242/dev.070771
Schonrock N, Ke YD, Humphreys D et al (2010) Neuronal microRNA deregulation in response to Alzheimer’s disease amyloid-beta. PLoS One. doi:10.1371/journal.pone.0011070
Zovoilis A, Agbemenyah HY, Agis-Balboa RC et al (2011) microRNA-34c is a novel target to treat dementias. EMBO J 30:4299–4308. doi:10.1038/emboj.2011.327
Im H-I, Kenny PJ (2012) MicroRNAs in neuronal function and dysfunction. Trends Neurosci 35:325–334. doi:10.1016/j.tins.2012.01.004
Delay C, Mandemakers W, Hébert SS (2012) MicroRNAs in Alzheimer’s disease. Neurobiol Dis 46:285–290. doi:10.1016/j.nbd.2012.01.003
Fischer A (2014) Targeting histone-modifications in Alzheimer’s disease. What is the evidence that this is a promising therapeutic avenue? Neuropharmacology 80:95–102. doi:10.1016/j.neuropharm.2014.01.038
Montesanto A, Dato S, Bellizzi D et al (2012) Epidemiological, genetic and epigenetic aspects of the research on healthy ageing and longevity. Immun Ageing 9:6. doi:10.1186/1742-4933-9-6
Cruickshanks HA, McBryan T, Nelson DM, Vanderkraats ND, Shah PP et al (2013) Senescent cells harbour features of the cancer epigenome. Nat Cell Biol 15:1495–1506. doi:10.1038/ncb2879
Salta E, Sierksma A, Vanden Eynden E, De Strooper B (2016) miR-132 loss de-represses ITPKB and aggravates amyloid and TAU pathology in Alzheimer’s brain. EMBO Mol Med 8:1005–1018. doi:10.15252/emmm.201606520
Zhang W, Li J, Suzuki K et al (2015) A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 348:1160–1163. doi:10.1126/science.aaa1356
Cheung I, Shulha HP, Jiang Y et al (2010) Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proc Natl Acad Sci U S A 107:8824–8829. doi:10.1073/pnas.1001702107
Liu L, Cheung TH, Charville GW et al (2013) Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep 4:189–204. doi:10.1016/j.celrep.2013.05.043
Pu M, Ni Z, Wang M et al (2015) Trimethylation of Lys36 on H3 restricts gene expression change during aging and impacts life span. Genes Dev 29:718–731. doi:10.1101/gad.254144.114
Southworth LK, Owen AB, Kim SK (2009) Aging mice show a decreasing correlation of gene expression within genetic modules. PLoS Genet 5:e1000776. doi:10.1371/journal.pgen.1000776
Numata S, Ye T, Hyde TM et al (2012) DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet 90:260–272. doi:10.1016/j.ajhg.2011.12.020
Hannum G, Guinney J, Zhao L et al (2013) Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 49:359–367. doi:10.1016/j.molcel.2012.10.016
Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:R115. doi:10.1186/gb-2013-14-10-r115
Klein HU, Bennett DA, de Jager PL (2016) The epigenome in Alzheimer’s disease: current state and approaches for a new path to gene discovery and understanding disease mechanism. Acta Neuropathol:1–12. doi:10.1007/s00401-016-1612-7
Horvath S, Mah V, Lu AT et al (2015) The cerebellum ages slowly according to the epigenetic clock. Aging (Albany NY) 7:294–306. doi:10.18632/aging.100742
Yang J, Yu L, Gaiteri C et al (2015) Association of DNA methylation in the brain with age in older persons is confounded by common neuropathologies. Int J Biochem Cell Biol 67:58–64. doi:10.1016/j.biocel.2015.05.009
Fuke C, Shimabukuro M, Petronis A et al (2004) Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet 68:196–204. doi:10.1046/j.1529-8817.2004.00081.x
Vanyushin BF, Nemirovsky LE, Klimenko VV et al (1973) The 5-methylcytosine in DNA of rats. Tissue and age specificity and the changes induced by hydrocortisone and other agents. Gerontologia 19:138–152
Wilson VL, Smith RA, Ma S, Cutler RG (1987) Genomic 5-methyldeoxycytidine decreases with age. J Biol Chem 262:9948–9951
Mastroeni D, Grover A, Delvaux E et al (2010) Epigenetic changes in Alzheimer’s disease: decrements in DNA methylation. Neurobiol Aging 31:2025–2037. doi:10.1016/j.neurobiolaging.2008.12.005
Corder EH, Saunders AM, Strittmatter WJ et al (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261:921–923. doi:10.1126/science.8346443
Wang SC, Oeize B, Schumacher A (2008) Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS One. doi:10.1371/journal.pone.0002698
Bernstein AI, Lin Y, Street RC, et al. (2016) 5-Hydroxymethylation-associated epigenetic modifiers of Alzheimer’s disease modulate tau-induced neurotoxicity. 0:1–14. doi: 10.1093/hmg/ddw109
Shu L, Sun W, Li L et al (2016) Genome-wide alteration of 5-hydroxymenthylcytosine in a mouse model of Alzheimer’s disease. BMC Genomics 17:381. doi:10.1186/s12864-016-2731-1
Song C-X, Szulwach KE, Fu Y et al (2011) Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol 29:68–72. doi:10.1038/nbt.1732
Irier HA, Jin P (2012) Dynamics of DNA methylation in aging and Alzheimer’s disease. DNA Cell Biol 31:S-42–S-48. doi:10.1089/dna.2011.1565
Lovestone S, Reynolds CH (1997) The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative process. Neuroscience 78:309–324. doi:10.1016/S0306-4522(96)00577-5
Alonso A, Zaidi T, Novak M et al (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A 98:6923–6928. doi:10.1073/pnas.121119298
Dourlen P, Fernandez-Gomez FJ, Dupont C et al (2016) Functional screening of Alzheimer risk loci identifies PTK2B as an in vivo modulator and early marker of tau pathology. Mol Psychiatry:1–10. doi:10.1038/mp.2016.59
Tohgi H, Utsugisawa K, Nagane Y et al (1999) The methylation status of cytosines in a tau gene promoter region alters with age to downregulate transcriptional activity in human cerebral cortex. Neurosci Lett 275:89–92
Sontag E, Nunbhakdi-Craig V, Sontag J-M et al (2007) Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci 27:2751–2759. doi:10.1523/JNEUROSCI.3316-06.2007
Nicolia V, Fuso A, Cavallaro RA, Di A (2010) B vitamin deficiency promotes tau phosphorylation through regulation of GSK3β and PP2A. 19:895–907. doi:10.3233/JAD-2010-1284
Popkie AP, Zeidner LC, Albrecht AM et al (2010) Phosphatidylinositol 3-kinase (PI3K) signaling via glycogen synthase kinase-3 (Gsk-3) regulates DNA methylation of imprinted loci. J Biol Chem 285:41337–41347. doi:10.1074/jbc.M110.170704
Zhou XW, Gustafsson JA, Tanila H et al (2008) Tau hyperphosphorylation correlates with reduced methylation of protein phosphatase 2A. Neurobiol Dis 31:386–394. doi:10.1016/j.nbd.2008.05.013
Yoon SY, Choi HI, Choi JE et al (2007) Methotrexate decreases PP2A methylation and increases tau phosphorylation in neuron. Biochem Biophys Res Commun 363:811–816. doi:10.1016/j.bbrc.2007.09.060
Zhang CE, Tian Q, Wei W et al (2008) Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging 29:1654–1665. doi:10.1016/j.neurobiolaging.2007.04.015
Alzheimer’s Association. http://www.alz.org/alzheimers_disease_causes_risk_factors.asp. Accessed 4 Jun 2016
Mastroeni D, Grover A, Delvaux E et al (2011) Epigenetic mechanisms in Alzheimer’s disease. Neurobiol Aging 32:1161–1180. doi:10.1016/j.neurobiolaging.2010.08.017
Liu H, Li W, Zhao S et al (2016) Folic acid attenuates the effects of amyloid β oligomers on DNA methylation in neuronal cells. Eur J Nutr 55:1849–1862. doi:10.1007/s00394-015-1002-2
Sibani S, Melnyk S, Pogribny IP et al (2002) Studies of methionine cycle intermediates (SAM, SAH), DNA methylation and the impact of folate deficiency on tumor numbers in Min mice. Carcinogenesis 23:61–65. doi:10.1093/carcin/23.1.61
Trasler J, Deng L, Melnyk S et al (2003) Impact of Dnmt1 deficiency, with and without low folate diets, on tumor numbers and DNA methylation in min mice. Carcinogenesis 24:39–45. doi:10.1093/carcin/24.1.39
Bottiglieri T, Godfrey P, Flynn T et al (1990) Cerebrospinal fluid S-adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S-adenosylmethionine. J Neurol Neurosurg Psychiatry 53:1096–1098. doi:10.1136/jnnp.53.12.1096
Morrison LD, Smith DD, Kish SJ (1996) Brain S-adenosylmethionine levels are severely decreased in Alzheimer’s disease. J Neurochem 67:1328–1331. doi:10.1046/j.1471-4159.1996.67031328.x
Serot JM, Christmann D, Dubost T et al (2001) CSF-folate levels are decreased in late-onset AD patients. J Neural Transm 108:93–99. doi:10.1007/s007020170100
Kennedy BP, Bottiglieri T, Arning E et al (2004) Elevated S-adenosylhomocysteine in Alzheimer brain: influence on methyltransferases and cognitive function. J Neural Transm 111:547–567. doi:10.1007/s00702-003-0096-5
Coppedè F, Tannorella P, Pezzini I et al (2012) Folate, homocysteine, vitamin B12, and polymorphisms of genes participating in one-carbon metabolism in late-onset Alzheimer’s disease patients and healthy controls. Antioxid Redox Signal 17:195–204. doi:10.1089/ars.2011.4368
Obeid R, McCaddon A, Herrmann W (2007) The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. 1590–1606
van Groen T (2010) DNA methylation and Alzheimer’s disease. In: Epigenetics of aging. New York, Springer New York, pp 315–326
Barthet G, Georgakopoulos A, Robakis NK (2012) Cellular mechanisms of γ-secretase substrate selection, processing and toxicity. Prog Neurobiol 98:166–175. doi:10.1016/j.pneurobio.2012.05.006
Hollingworth P, Harold D, Jones L et al (2011) Alzheimer’s disease genetics: current knowledge and future challenges. Int J Geriatr Psychiatry 26:793–802. doi:10.1002/gps.2628
Migliore L, Coppedè F (2009) Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res—Fundam Mol Mech Mutagen 667:82–97. doi:10.1016/j.mrfmmm.2008.10.011
Wolfe MS, Xia W, Ostaszewski BL et al (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 398:513–517. doi:10.1038/19077
Fuso A, Seminara L, Cavallaro RA et al (2005) S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci 28:195–204. doi:10.1016/j.mcn.2004.09.007
Scarpa S, Fuso A, D’Anselmi F, Cavallaro RA (2003) Presenilin 1 gene silencing by S-adenosylmethionine: a treatment for Alzheimer disease? FEBS Lett 541:145–148. doi:10.1016/S0014-5793(03)00277-1
Piaceri I, Raspanti B, Tedde A et al (2015) Epigenetic modifications in Alzheimer’s disease: cause or effect? J Alzheimers Dis 43:1169–1173. doi:10.3233/JAD-141452
Liu W, Liu C, Zhu J et al (2012) MicroRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer’s-associated pathogenesis in SAMP8 mice. Neurobiol Aging 33:522–534. doi:10.1016/j.neurobiolaging.2010.04.034
Tohgi H, Utsugisawa K, Nagane Y et al (1999) Reduction with age in methylcytosine in the promoter region −224∼−101 of the amyloid precursor protein gene in autopsy human cortex. Mol Brain Res 70:288–292. doi:10.1016/S0169-328X(99)00163-1
Barrachina M, Ferrer I (2009) DNA methylation of Alzheimer disease and tauopathy-related genes in postmortem brain. 68:880–891. doi:10.1097/NEN.0b013e3181af2e46
Brohede J, Rinde M, Winblad B, Graff C (2010) A DNA methylation study of the amyloid precursor protein gene in several brain regions from patients with familial Alzheimer disease. J Neurogenet 24:179–181. doi:10.3109/01677063.2010.503978
Fuso A, Nicolia V, Cavallaro RA et al (2008) B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-β deposition in mice. Mol Cell Neurosci 37:731–746. doi:10.1016/j.mcn.2007.12.018
Lin H-C, Hsieh H-M, Chen Y-H, Hu M-L (2009) S-Adenosylhomocysteine increases beta-amyloid formation in BV-2 microglial cells by increased expressions of beta-amyloid precursor protein and presenilin 1 and by hypomethylation of these gene promoters. Neurotoxicology 30:622–627. doi:10.1016/j.neuro.2009.03.011
Cong L, Jia J, Qin W et al (2014) Genome-wide analysis of DNA methylation in an APP/PS1 mouse model of Alzheimer’s disease. Acta Neurol Belg 114:195–206. doi:10.1007/s13760-013-0267-6
Chen KL, Wang SSS, Yang YY et al (2009) The epigenetic effects of amyloid-β1-40 on global DNA and neprilysin genes in murine cerebral endothelial cells. Biochem Biophys Res Commun 378:57–61. doi:10.1016/j.bbrc.2008.10.173
Carboni L, Lattanzio F, Candeletti S et al (2015) Peripheral leukocyte expression of the potential biomarker proteins Bdnf, Sirt1, and Psen1 is not regulated by promoter methylation in Alzheimer’s disease patients. Neurosci Lett 605:44–48. doi:10.1016/j.neulet.2015.08.012
Offe K (2006) The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci 26:1596–1603. doi:10.1523/JNEUROSCI.4946-05.2006
Furuya TK, Da Silva PNO, Payão SLM et al (2012) SORL1 and SIRT1 mRNA expression and promoter methylation levels in aging and Alzheimer’s disease. Neurochem Int 61:973–975. doi:10.1016/j.neuint.2012.07.014
Müerköster SS, Werbing V, Koch D et al (2008) Role of myofibroblasts in innate chemoresistance of pancreatic carcinoma-epigenetic downregulation of caspases. Int J Cancer 123:1751–1760. doi:10.1002/ijc.23703
Wilson AG (2008) Epigenetic regulation of gene expression in the inflammatory response and relevance to common diseases. J Periodontol 79:1514–1519. doi:10.1902/jop.2008.080172
Xiong M, Zhang T, Zhang LM et al (2008) Caspase inhibition attenuates accumulation of β-amyloid by reducing β-secretase production and activity in rat brains after stroke. Neurobiol Dis 32:433–441. doi:10.1016/j.nbd.2008.08.007
Sommer G, Kralisch S, Lipfert J et al (2009) Amyloid precursor protein expression is induced by tumor necrosis factor alpha in 3T3-L1 adipocytes. J Cell Biochem 108:1418–1422. doi:10.1002/jcb.22382
Drzezga A, Grimmer T, Henriksen G (2009) Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. i:1487–1494. doi:10.1212/WNL.0b013e3181a2e8d0
Caesar I, Gandy S (2012) Evidence that an APOE epsilon4 “double whammy” increases risk for Alzheimer’s disease. BMC Med 10:36. doi:10.1186/1741-7015-10-36
Cruchaga C, Kauwe JSK, Nowotny P et al (2012) Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer’s disease. Hum Mol Genet 21:4558–4571. doi:10.1093/hmg/dds296
Castellano JM, Kim J, Stewart FR et al (2011) Human APOE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med 3:89ra57. doi:10.1126/scitranslmed.3002156
de Bem CMBE, Pezzi JC, Borba EM et al (2016) The synergistic risk effect of apolipoprotein ε4 and DNA (cytosine-5-)-methyltransferase 3 beta (DNMT3B) haplotype for Alzheimer’s disease. Mol Biol Rep 43:653–658. doi:10.1007/s11033-016-3999-6
Yu JT, Tan L (2012) The role of clusterin in Alzheimer’s disease: pathways, pathogenesis, and therapy. Mol Neurobiol 45:314–326. doi:10.1007/s12035-012-8237-1
Schrijvers EMC, Koudstaal PJ, Hofman A, Breteler MMB (2011) Plasma clusterin and the risk of Alzheimer disease. JAMA 305:1322–1326. doi:10.1001/jama.2011.381
Thambisetty M, Simmons A, Velayudhan L et al (2010) Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry 67:739–748. doi:10.1001/archgenpsychiatry.2010.78
Rauhala HE, Porkka KP, Saramäki OR et al (2008) Clusterin is epigenetically regulated in prostate cancer. Int J Cancer 123:1601–1609. doi:10.1002/ijc.23658
Nuutinen T, Suuronen T, Kyrylenko S et al (2005) Induction of clusterin/apoJ expression by histone deacetylase inhibitors in neural cells. Neurochem Int 47:528–538. doi:10.1016/j.neuint.2005.07.007
Suuronen T, Nuutinen T, Ryhänen T et al (2007) Epigenetic regulation of clusterin/apolipoprotein J expression in retinal pigment epithelial cells. Biochem Biophys Res Commun 357:397–401. doi:10.1016/j.bbrc.2007.03.135
Wang J, Yu J-T, Tan M-S et al (2013) Epigenetic mechanisms in Alzheimer’s disease: implications for pathogenesis and therapy. Ageing Res Rev 12:1024–1041. doi:10.1016/j.arr.2013.05.003
Ding Q (2005) Ribosome dysfunction is an early event in Alzheimer’s disease. J Neurosci 25:9171–9175. doi:10.1523/JNEUROSCI.3040-05.2005
Pietrzak M, Rempala G, Nelson PT, et al. (2011) Epigenetic silencing of nucleolar rRNA genes in Alzheimer’s disease. 6:1–10. doi:10.1371/journal.pone.0022585
Pietrzak M, Rempala GA, Nelson PT, Hetman M (2016) Non-random distribution of methyl-CpG sites and non-CpG methylation in the human rDNA promoter identified by next generation bisulfite sequencing. Gene 585:35–43. doi:10.1016/j.gene.2016.03.028
Hansmannel F, Lendon C, Pasquier F et al (2009) Is the ornithine transcarbamylase gene a genetic determinant of Alzheimer’s disease? Neurosci Lett 449:76–80. doi:10.1016/j.neulet.2008.10.081
Bensemain F, Hot D, Ferreira S et al (2009) Evidence for induction of the ornithine transcarbamylase expression in Alzheimer’s disease. Mol Psychiatry 14:106–116. doi:10.1038/sj.mp.4002089
Kudriashova IB, Kirnos MD, Vaniushin BF (1976) DNA-methylase activities from animal mitochondria and nuclei: different specificity of DNA methylation. Biokhimiia (Moscow, Russ) 41:1968–1977
Nass MMK (1973) Differential methylation of mitochondrial and nuclear DNA in cultured mouse, hamster and virus-transformed hamster cells in vivo and in vitro methylation. J Mol Biol 80:155–175. doi:10.1016/0022-2836(73)90239-8
Reis RJS, Goldstein S (1983) Mitochondrial DNA in mortal and immortal human cells. J Biol Chem 258:9078–9085
Shock LS, Thakkar PV, Peterson EJ et al (2011) DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proc Natl Acad Sci 108:3630–3635. doi:10.1073/pnas.1012311108
Choi Y-S, Hoon Jeong J, Min H-K et al (2011) Shot-gun proteomic analysis of mitochondrial D-loop DNA binding proteins: identification of mitochondrial histones. Mol BioSyst 7:1523. doi:10.1039/c0mb00277a
Barrey E, Saint-Auret G, Bonnamy B et al (2011) Pre-microRNA and mature microRNA in human mitochondria. PLoS One 6:e20220. doi:10.1371/journal.pone.0020220
Chestnut BA, Chang Q, Price A et al (2011) Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci 31:16619–16636. doi:10.1523/JNEUROSCI.1639-11.2011
Dzitoyeva S, Chen H, Manev H (2012) Effect of aging on 5-hydroxymethylcytosine in brain mitochondria. Neurobiol Aging 33:2881–2891. doi:10.1016/j.neurobiolaging.2012.02.006
Iacobazzi V, Castegna A, Infantino V, Andria G (2013) Mitochondrial DNA methylation as a next-generation biomarker and diagnostic tool. Mol Genet Metab 110:25–34. doi:10.1016/j.ymgme.2013.07.012
Manev H, Dzitoyeva S, Chen H (2012) Mitochondrial DNA: a blind spot in neuroepigenetics. 3:107–115. doi: 10.1515/bmc-2011-0058
Blanch M, Mosquera JL, Ansoleaga B et al (2016) Altered mitochondrial DNA methylation pattern in Alzheimer disease-related pathology and in Parkinson disease. Am J Pathol 186:385–397. doi:10.1016/j.ajpath.2015.10.004
Halder R, Hennion M, Vidal RO et al (2015) DNA methylation changes in plasticity genes accompany the formation and maintenance of memory. Nat Neurosci 19:102–110. doi:10.1038/nn.4194
Liu HC, Hu CJ, Tang YC, Chang JG (2008) A pilot study for circadian gene disturbance in dementia patients. Neurosci Lett 435:229–233. doi:10.1016/j.neulet.2008.02.041
Bollati V, Galimberti D, Pergoli L et al (2011) DNA methylation in repetitive elements and Alzheimer disease. Brain Behav Immun 25:1078–1083. doi:10.1016/j.bbi.2011.01.017
Khan AA, Mao XO, Banwait S et al (2007) Neuroglobin attenuates beta-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc Natl Acad Sci U S A 104:19114–19119. doi:10.1073/pnas.0706167104
Zhang W, Tian Z, Sha S et al (2011) Functional and sequence analysis of human neuroglobin gene promoter region. Biochim Biophys Acta 1809:236–244. doi:10.1016/j.bbagrm.2011.02.003 %/ 2011 Elsevier B.V. All rights reserved
Guan J-Z, Guan W-P, Maeda T, Makino N (2012) Effect of vitamin E administration on the elevated oxygen stress and the telomeric and subtelomeric status in Alzheimer’s disease. Gerontology 58:62–69. doi:10.1159/000327821
Rao JS, Keleshian VL, Klein S, Rapoport SI (2012) Epigenetic modifications in frontal cortex from Alzheimer’s disease and bipolar disorder patients. Transl Psychiatry 2:e132. doi:10.1038/tp.2012.55
Roth ED, Roth TL, Money KM et al (2015) DNA methylation regulates neurophysiological spatial representation in memory formation. Neuroepigenetics 2:1–8. doi:10.1016/j.nepig.2015.03.001
Silva PN, Furuya TK, Sampaio Braga I et al (2013) CNP and DPYSL2 mRNA expression and promoter methylation levels in brain of Alzheimer’s disease patients. J Alzheimers Dis 33:349–355. doi:10.3233/JAD-2012-121192
Silva PNO, Gigek CO, Leal MF et al (2008) Promoter methylation analysis of SIRT3, SMARCA5, HTERT and CDH1 genes in aging and Alzheimer’s disease. J Alzheimers Dis 13:173–176
Furuya TK, Silva PNO, Payão SLM et al (2012) Analysis of SNAP25 mRNA expression and promoter DNA methylation in brain areas of Alzheimer’s disease patients. Neuroscience 220:41–46. doi:10.1016/j.neuroscience.2012.06.035
Moreira PR, Guimarães MM, ALS G et al (2009) Methylation of P16, P21, P27, RB1 and P53 genes in odontogenic keratocysts. J Oral Pathol Med 38:99–103. doi:10.1111/j.1600-0714.2008.00718.x
Tschöp K, Engeland K (2007) Cell cycle-dependent transcription of cyclin B2 is influenced by DNA methylation but is independent of methylation in the CDE and CHR elements. FEBS J 274:5235–5249. doi:10.1111/j.1742-4658.2007.06045.x
Jee CD, Lee HS, Bae SI et al (2005) Loss of caspase-1 gene expression in human gastric carcinomas and cell lines. Int J Oncol 26:1265–1271
Robertson KD, Jones PA (1998) The human ARF cell cycle regulatory gene promoter is a CpG island which can be silenced by DNA methylation and down-regulated by wild-type p53. Mol Cell Biol 18:6457–6473
Siegmund KD, Connor CM, Campan M et al (2007) DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One. doi:10.1371/journal.pone.0000895
Zukin S (2009) Epigenetics. Alzheimers Dement 5:P146–P147. doi:10.1016/j.jalz.2009.05.502
Sanchez-Mut JV, Aso E, Panayotis N et al (2013) DNA methylation map of mouse and human brain identifies target genes in Alzheimer’s disease. Brain 136:3018–3027. doi:10.1093/brain/awt237
Sanchez-Mut JV, Heyn H, Vidal E et al (2016) Human DNA methylomes of neurodegenerative diseases show common epigenomic patterns. Transl Psychiatry 6:e718. doi:10.1038/tp.2015.214
De Jager PL, Srivastava G, Lunnon K et al (2014) Alzheimer’s disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci 17:1156–1163. doi:10.1038/nn.3786 http://www.nature.com/neuro/journal/v17/n9/abs/nn.3786.html#supplementary-information
Lunnon K, Smith R, Hannon E et al (2014) Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer’s disease. Nat Neurosci 17:1164–1170. doi:10.1038/nn.3782
Shinagawa S, Kobayashi N, Nagata T et al (2016) DNA methylation in the NCAPH2/LMF2 promoter region is associated with hippocampal atrophy in Alzheimer’s disease and amnesic mild cognitive impairment patients. Neurosci Lett 629:33–37. doi:10.1016/j.neulet.2016.06.055
Kobayashi N, Shinagawa S, Nagata T et al (2016) Development of biomarkers based on DNA methylation in the NCAPH2/LMF2 promoter region for diagnosis of Alzheimer’s disease and amnesic mild cognitive impairment. PLoS One 11:1–12. doi:10.1371/journal.pone.0146449
Di Francesco A, Arosio B, Falconi A et al (2015) Global changes in DNA methylation in Alzheimer’s disease peripheral blood mononuclear cells. Brain Behav Immun 45:139–144. doi:10.1016/j.bbi.2014.11.002
Ferri E, Arosio B, D’Addario C et al (2016) Gene promoter methylation and expression of Pin1 differ between patients with frontotemporal dementia and Alzheimer’s disease. J Neurol Sci 362:283–286. doi:10.1016/j.jns.2016.02.004
Ji H, Wang Y, Jiang D et al (2016) Elevated DRD4 promoter methylation increases the risk of Alzheimer’s disease in males. Mol Med Rep 14:2732–2738. doi:10.3892/mmr.2016.5560
Ma SL, Sang Tang NL, Wa Lam LC (2016) Association of gene expression and methylation of UQCRC1 to the predisposition of Alzheimer’s disease in a Chinese population. J Psychiatr Res 76:143–147. doi:10.1016/j.jpsychires.2016.02.010
Watson CT, Roussos P, Garg P et al (2016) Genome-wide DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer’s disease. Genome Med 8:5. doi:10.1186/s13073-015-0258-8
Moore DS The developing genome: an introduction to behavioral epigenetics
Sato T, Cesaroni M, Chung W et al (2016) Transcriptional selectivity of epigenetic therapy in cancer. Cancer Res. doi:10.1158/0008-5472.CAN-16-0834
Durga J, van Boxtel MP, Schouten EG et al (2007) Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 369:208–216. doi:10.1016/S0140-6736(07)60109-3
Haan MN, Miller JW, Aiello AE et al (2007) Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr 85:511–517
Werneke U, Turner T, Priebe S (2012) Complementary medicines in psychiatry: review of effectiveness and safety complementary medicines in psychiatry review of effectiveness and safety. 109–121. doi: 10.1192/bjp.188.2.109
Stahl SM (2010) Fooling mother nature: epigenetics and novel treatments for psychiatric disorders. CNS Spectr 15:358–365
Stahl SM (2010) Methylated spirits: epigenetic hypotheses of psychiatric disorders. CNS Spectr 15:220–230
Cao X-J, Huang S-H, Wang M et al (2008) S-adenosyl-L-methionine improves impaired hippocampal long-term potentiation and water maze performance induced by developmental lead exposure in rats. Eur J Pharmacol 595:30–34. doi:10.1016/j.ejphar.2008.07.061
Issa JPJ, Garcia-Manero G, Giles FJ et al (2004) Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 103:1635–1640. doi:10.1182/blood-2003-03-0687
Momparler RL, Bouffard DY, Momparler LF et al (1997) Pilot phase I-II study on 5-aza-2′-deoxycytidine (decitabine) in patients with metastatic lung cancer. Anti-Cancer Drugs 8:358–368
Kelly TK, De Carvalho DD, Jones PA (2010) Epigenetic modifications as therapeutic targets. Nat Biotechnol 28:1069–1078. doi:10.1038/nbt.1678
Kumar D, Aggarwal M, Kaas GA et al (2015) Tet1 oxidase regulates neuronal gene transcription, active DNA hydroxy-methylation, object location memory, and threat recognition memory HHS public access. Neuroepigenetics 4:12–27. doi:10.1016/j.nepig.2015.10.002
Su Y, Ryder J, Li B, et al. (2004) Lithium, a common drug for bipolar disorder treatment, regulates amyloid- precursor protein processing. 6899–6908
Qing H, He G, Ly PTT et al (2008) Valproic acid inhibits Aβ production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J Exp Med 205:2781–2789. doi:10.1084/jem.20081588
Kilgore M, Miller CA, Fass DM et al (2010) Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 35:870–880. doi:10.1038/npp.2009.197
Ricobaraza A, Cuadrado-Tejedor M, Pérez-Mediavilla A et al (2009) Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology 34:1721–1732. doi:10.1038/npp.2008.229
Ricobaraza A, Cuadrado-Tejedor M, Marco S et al (2012) Phenylbutyrate rescues dendritic spine loss associated with memory deficits in a mouse model of Alzheimer disease. Hippocampus 22:1040–1050. doi:10.1002/hipo.20883
Peleg S, Sananbenesi F, Zovoilis A, et al. (2010) Altered histone acetylation is associated with age-dependent memory impairment in mice. Science (80-. ) 328
Nuutinen T, Suuronen T, Kauppinen A, Salminen A (2010) Valproic acid stimulates clusterin expression in human astrocytes: implications for Alzheimer’s disease. Neurosci Lett. doi:10.1016/j.neulet.2010.03.041
Vecsey CG, Hawk JD, Lattal KM, et al. (2007) Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. J Neurosci 27
Ishimaru N, Fukuchi M, Hirai A et al (2010) Differential epigenetic regulation of BDNF and NT-3 genes by trichostatin A and 5-aza-2′-deoxycytidine in neuro-2a cells. Biochem Biophys Res Commun. doi:10.1016/j.bbrc.2010.02.139
Tian F, Marini AM, Lipsky RH (2010) Effects of histone deacetylase inhibitor trichostatin A on epigenetic changes and transcriptional activation of Bdnf promoter 1 by rat hippocampal neurons. Ann N Y Acad Sci 1199:186–193. doi:10.1111/j.1749-6632.2009.05175.x
Green KN, Steffan JS, Martinez-Coria H, et al. (2008) Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-Phosphotau. J Neurosci 28
Brahe C, Vitali T, Tiziano FD, et al. (2005) Phenylbutyrate increases SMN gene expression in spinal muscular atrophy patients. 256–259. doi:10.1038/sj.ejhg.5201320
Marks PA, Xu W-S (2009) Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem 107:600–608. doi:10.1002/jcb.22185
Marks PA (2010) The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin Investig Drugs 19:1049–1066. doi:10.1517/13543784.2010.510514
Salminen A, Tapiola T, Korhonen P, Suuronen T (1998) Neuronal apoptosis induced by histone deacetylase inhibitors. Mol Brain Res 61:203–206. doi:10.1016/S0169-328X(98)00210-1
Kelly-Sell MJ, Kim YH, Straus S et al (2012) The histone deacetylase inhibitor, romidepsin, suppresses cellular immune functions of cutaneous T-cell lymphoma patients. Am J Hematol 87:354–360. doi:10.1002/ajh.23112
Rossi LE, Avila DE, Spallanzani RG et al (2012) Histone deacetylase inhibitors impair NK cell viability and effector functions through inhibition of activation and receptor expression. J Leukoc Biol 91:321–331. doi:10.1189/JLB.0711339
Arrowsmith CH, Bountra C, Fish PV et al (2012) Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov 11:384–400. doi:10.1038/nrd3674
Trapp J, Meier R, Hongwiset D et al (2007) Structure–activity studies on suramin analogues as inhibitors of NAD+-dependent histone deacetylases (sirtuins). ChemMedChem 2:1419–1431. doi:10.1002/cmdc.200700003
Haggarty SJ, Koeller KM, Wong JC et al (2003) Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci 100:4389–4394. doi:10.1073/PNAS.0430973100
Ding H, Dolan PJ, Johnson GVW (2008) Histone deacetylase 6 interacts with the microtubule-associated protein tau. J Neurochem 106:2119–2130. doi:10.1111/j.1471-4159.2008.05564.x
Fang M, Wang J, Zhang X et al (2012) The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicol Lett 209:94–105. doi:10.1016/j.toxlet.2011.11.032
Zhu H-C, Wang L-M, Wang M et al (2012) MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting BACE1. Brain Res Bull 88:596–601. doi:10.1016/j.brainresbull.2012.05.018
Junn E, Mouradian MM (2012) MicroRNAs in neurodegenerative diseases and their therapeutic potential. Pharmacol Ther 133:142–150. doi:10.1016/j.pharmthera.2011.10.002
Alvarez-Erviti L, Seow Y, Yin H et al (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. doi:10.1038/nbt.1807
Lakhal S, El Andaloussi S, O’Loughlin AJ, et al. (2013) RNAi therapeutic delivery by exosomes. Springer US, pp 185–205
Kuhn DE, Nuovo GJ, Terry AV et al (2010) Chromosome 21-derived microRNAs provide an etiological basis for aberrant protein expression in human down syndrome brains. J Biol Chem 285:1529–1543. doi:10.1074/jbc.M109.033407
Bakulski KM, Dolinoy DC, Sartor MA et al (2012) Genome-wide DNA methylation differences between late-onset Alzheimer’s disease and cognitively normal controls in human frontal cortex. J Alzheimers Dis 29:571–588. doi:10.3233/JAD-2012-111223
Acknowledgements
We thank Mr. Adil Farooq Lodhi (Department of Microbiology, Hazara University, Manshera, Pakistan) and Ms. Lucienne Duru (School of Life Science, Beijing Institute of Technology, Beijing, China) for their useful suggestions and support, which helped us completing this work. This work was supported by National Natural Science Foundation of China (NSFC 81671268) and Ministry of Science and Technology China (No. 2013YQ03059514).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Qazi, T.J., Quan, Z., Mir, A. et al. Epigenetics in Alzheimer’s Disease: Perspective of DNA Methylation. Mol Neurobiol 55, 1026–1044 (2018). https://doi.org/10.1007/s12035-016-0357-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0357-6