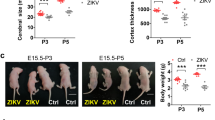
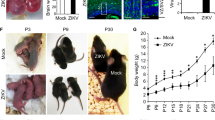
Abstract
The Zika virus (ZIKV) outbreak that occurred in the northeast of Brazil in 2015 led to alarming numbers of babies born with microcephaly in this region. Since then, several studies have evaluated the relationship between ZIKV infection and development of the malformation although the specific mechanistic interaction between ZIKV and human physiological processes that ultimately manifest as microcephaly remains debated. Importantly, most current studies did not consider the specificities of the biology and life cycle of ZIKV. As a consequence, specificities of the infection on the developing central nervous system (CNS) were frequently disregarded. In order to begin to address this important gap in our knowledge, we have collated and critically reviewed the existing evidence in this area to identify any emerging consensus on this topic and thereafter describe possible mechanisms by which ZIKV infection could interfere with specific processes of CNS development, such as neuronal proliferation, and the complex interactions of immature neurons with radial glial cells. With this, we were able to present the current knowledge on this important topic in the neurobiology field.



Similar content being viewed by others
References
Mukhopadhyay S, Kuhn RJ, Rossmann MG (2005) A structural perspective of the Flavivirus life cycle. Nat Rev Microbiol 3(1):13–22
Sips GJ, Wilschut J, Smit JM (2012) Neuroinvasive Flavivirus infections. Rev Med Virol 22(2):69–87
Kuno G et al (1998) Phylogeny of the genus Flavivirus. J Virol 72(1):73–83
Chambers TJ et al (1990) Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44:649–688
Huang YJ et al (2014) Flavivirus-mosquito interactions. Viruses 6(11):4703–4730
Nicholson BL, White KA (2014) Functional long-range RNA-RNA interactions in positive-strand RNA viruses. Nat Rev Microbiol 12(7):493–504
Shives KD et al (2014) West Nile virus-induced activation of mammalian target of rapamycin complex 1 supports viral growth and viral protein expression. J Virol 88(16):9458–9471
Harris, E., et al., Molecular biology of Flaviviruses. Novartis Found Symp, 2006. 277: p. 23–39; discussion 40, 71–3, 251–3.
Faye O et al (2014) Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 8(1):e2636
Ye J et al (2013) Immune evasion strategies of flaviviruses. Vaccine 31(3):461–471
Mackenzie JM et al (1998) Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology 245(2):203–215
Teo CS, Chu JJ (2014) Cellular vimentin regulates construction of dengue virus replication complexes through interaction with NS4A protein. J Virol 88(4):1897–1913
Adibi, J.J., et al., Teratogenic effects of the Zika virus and the role of the placenta. Lancet, 2016.
Lazear, H.M., et al., A mouse model of Zika virus pathogenesis. Cell Host Microbe, 2016.
Hamel R et al (2015) Biology of Zika virus infection in human skin cells. J Virol 89(17):8880–8896
Quicke KM et al (2016) Zika virus infects human placental macrophages. Cell Host Microbe 20(1):83–90
Tappe, D., et al., Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol, 2015.
Stettler, K., et al., Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science, 2016.
Priyamvada L et al (2016) Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 113(28):7852–7857
Dejnirattisai, W., et al., Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol, 2016.
Barba-Spaeth G et al (2016) Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536(7614):48–53
Whitehead SS et al (2007) Prospects for a dengue virus vaccine. Nat Rev Microbiol 5(7):518–528
Murphy BR, Whitehead SS (2011) Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol 29:587–619
Diamond MS (2003) Evasion of innate and adaptive immunity by flaviviruses. Immunol Cell Biol 81(3):196–206
Noronha, L., et al., Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz, 2016.
Bayer, A., et al., Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe, 2016.
Miner JJ et al (2016) Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165(5):1081–1091
Aliota MT et al (2016) Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl Trop Dis 10(4):e0004682
Robbins JR, Bakardjiev AI (2012) Pathogens and the placental fortress. Curr Opin Microbiol 15(1):36–43
Delorme-Axford E, Sadovsky Y, Coyne CB (2014) The placenta as a barrier to viral infections. Annu Rev Virol 1(1):133–146
Shao Q et al (2016) Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 143(22):4127–4136
Mercuri E et al (2000) Head growth in infants with hypoxic-ischemic encephalopathy: correlation with neonatal magnetic resonance imaging. Pediatrics 106(2 Pt 1):235–243
Petraglia F, Imperatore A, Challis JR (2010) Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev 31(6):783–816
Witteveen JS et al (2013) Lack of serotonin reuptake during brain development alters rostral raphe-prefrontal network formation. Front Cell Neurosci 7:143
Vitalis T, Parnavelas JG (2003) The role of serotonin in early cortical development. Dev Neurosci 25(2–4):245–256
Vitalis T, Ansorge MS, Dayer AG (2013) Serotonin homeostasis and serotonin receptors as actors of cortical construction: special attention to the 5-HT3A and 5-HT6 receptor subtypes. Front Cell Neurosci 7:93
Montiel JF, Kaune H, Maliqueo M (2013) Maternal-fetal unit interactions and eutherian neocortical development and evolution. Front Neuroanat 7:22
Fietz SA et al (2012) Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc Natl Acad Sci U S A 109(29):11836–11841
Yawno T et al (2009) Role of neurosteroids in regulating cell death and proliferation in the late gestation fetal brain. Neuroscience 163(3):838–847
Nicol MB, Hirst JJ, Walker D (1999) Effects of pregnanolone on behavioural parameters and the responses to GABA(a) receptor antagonists in the late gestation fetal sheep. Neuropharmacology 38(1):49–63
Cucullo L (2009) Prenatal development of the human blood-brain barrier. In: Janigro D (ed) Mammalian brain development. Humana Press, New York, pp. 53–75
Neal JW (2014) Flaviviruses are neurotropic, but how do they invade the CNS? J Infect 69(3):203–215
McGavern DB, Kang SS (2011) Illuminating viral infections in the nervous system. Nat Rev Immunol 11(5):318–329
Dahm T et al (2016) Neuroinvasion and inflammation in viral central nervous system infections. Mediat Inflamm 2016:8562805
Spooner RA et al (2006) Retrograde transport pathways utilised by viruses and protein toxins. Virol J 3:26
Ramos-Castaneda J et al (1997) A 65-kDa trypsin-sensible membrane cell protein as a possible receptor for dengue virus in cultured neuroblastoma cells. J Neurovirol 3(6):435–440
Chu JJ, Ng ML (2003) Characterization of a 105-kDa plasma membrane associated glycoprotein that is involved in West Nile virus binding and infection. Virology 312(2):458–469
Das S et al (2009) Heat shock protein 70 on Neuro2a cells is a putative receptor for Japanese encephalitis virus. Virology 385(1):47–57
Cugola, F.R., et al., The Brazilian Zika virus strain causes birth defects in experimental models. Nature, 2016.
Dang, J., et al., Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell, 2016.
Garcez, P.P., et al., Zika virus impairs growth in human neurospheres and brain organoids. Science, 2016.
Li, C., et al., Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell, 2016.
Wu, K.Y., et al., Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res, 2016.
Garcez, P.P., et al., Combined proteome and transcriptome analyses reveal that Zika virus circulating in Brazil alters cell cycle and neurogenic programmes in human neurospheres. 2016, PeerJ Preprints.
Nowakowski TJ et al (2016) Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 18(5):591–596
Hanners, N.W., et al., Western Zika virus in human fetal neural progenitors persists long term with partial cytopathic and limited immunogenic effects. Cell Reports, 2016.
Tang, H., et al., Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell, 2016.
Garcez PP et al (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352(6287):816–818
Dowall SD et al (2016) A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis 10(5):e0004658
Bell TM, Field EJ, Narang HK (1971) Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch 35(2):183–193
Driggers RW et al (2016) Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 374(22):2142–2151
Culjat, M., et al., Clinical and imaging findings in an infant with Zika embryopathy. Clin Infect Dis, 2016.
Hazin AN et al (2016) Computed tomographic findings in microcephaly associated with Zika virus. N Engl J Med 374(22):2193–2195
Moron AF et al (2016) Microcephaly associated with maternal Zika virus infection. BJOG 123(8):1265–1269
Szelenyi J (2001) Cytokines and the central nervous system. Brain Res Bull 54(4):329–338
Probert L (2015) TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience 302:2–22
Benedict CA, Norris PS, Ware CF (2002) To kill or be killed: viral evasion of apoptosis. Nat Immunol 3(11):1013–1018
Griffin DE (2003) Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol 3(6):493–502
Blazquez AB et al (2014) Stress responses in Flavivirus-infected cells: activation of unfolded protein response and autophagy. Front Microbiol 5:266
Dreux M, Chisari FV (2010) Viruses and the autophagy machinery. Cell Cycle 9(7):1295–1307
Tetro, J.A., Zika and microcephaly: causation, correlation, or coincidence? Microbes Infect, 2016.
Jheng JR, Ho JY, Horng JT (2014) ER stress, autophagy, and RNA viruses. Front Microbiol 5:388
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115(10):2656–2664
Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15(4):233–249
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7(12):1013–1030
Marino G et al (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15(2):81–94
Verfaillie T et al (2010) Linking ER stress to autophagy: potential implications for cancer therapy. Int J Cell Biol 2010:930509
Kim J et al (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141
Liang, Q., et al., Zika virus NS4A and NS4B proteins deregulate Akt-mTOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell, 2016.
Maiuri MC et al (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8(9):741–752
Li J, Yuan J (2008) Caspases in apoptosis and beyond. Oncogene 27(48):6194–6206
Bhandary B et al (2012) An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci 14(1):434–456
Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4(7):552–565
Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27(50):6434–6451
Feng Y et al (2014) The machinery of macroautophagy. Cell Res 24(1):24–41
Urrego D et al (2014) Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond Ser B Biol Sci 369(1638):20130094
Herrup K, Yang Y (2007) Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci 8(5):368–378
Bloom J, Cross FR (2007) Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol 8(2):149–160
Hindley C, Philpott A (2012) Co-ordination of cell cycle and differentiation in the developing nervous system. Biochem J 444(3):375–382
Elias LA, Wang DD, Kriegstein AR (2007) Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448(7156):901–907
Elias LA, Kriegstein AR (2008) Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci 31(5):243–250
Naus CC, Aftab Q, Sin WC (2016) Common mechanisms linking connexin43 to neural progenitor cell migration and glioma invasion. Semin Cell Dev Biol 50:59–66
Dermietzel R et al (1989) Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci U S A 86(24):10148–10152
Yamamoto T et al (1992) Differential anatomical and cellular patterns of connexin43 expression during postnatal development of rat brain. Brain Res Dev Brain Res 66(2):165–180
Cina C et al (2007) Expression of connexins in embryonic mouse neocortical development. J Comp Neurol 504(3):298–313
Matsuuchi L, Naus CC (2013) Gap junction proteins on the move: connexins, the cytoskeleton and migration. Biochim Biophys Acta 1828(1):94–108
McLeod TL, Bechberger JF, Naus CC (2001) Determination of a potential role of the CCN family of growth regulators in connexin43 transfected C6 glioma cells. Cell Commun Adhes 8(4–6):441–445
Giepmans BN et al (2001) Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11(17):1364–1368
Xu X et al (2001) Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol 154(1):217–230
Cina C et al (2009) Involvement of the cytoplasmic C-terminal domain of connexin43 in neuronal migration. J Neurosci 29(7):2009–2021
Nadarajah B et al (1997) Differential expression of connexins during neocortical development and neuronal circuit formation. J Neurosci 17(9):3096–3111
Fushiki S et al (2003) Changes in neuronal migration in neocortex of connexin43 null mutant mice. J Neuropathol Exp Neurol 62(3):304–314
Wiencken-Barger AE et al (2007) A role for Connexin43 during neurodevelopment. Glia 55(7):675–686
Liu X et al (2010) Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J Neurosci 30(12):4197–4209
Oyamada M, Oyamada Y, Takamatsu T (2005) Regulation of connexin expression. Biochim Biophys Acta 1719(1–2):6–23
Melian EB et al (2010) NS1' of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frameshifting and plays a role in viral neuroinvasiveness. J Virol 84(3):1641–1647
Song, H., et al., Zika virus NS1 structure reveals diversity of electrostatic surfaces among flaviviruses. Nat Struct Mol Biol, 2016.
Zhu Z et al (2016) Comparative genomic analysis of pre-epidemic and epidemic zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg Microbes Infect 5:e22
Eyer L et al (2016) Nucleoside inhibitors of Zika virus. J Infect Dis 214(5):707–711
Carneiro BM et al (2016) The green tea molecule EGCG inhibits Zika virus entry. Virology 496:215–218
Barrows NJ et al (2016) A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe 20(2):259–270
Morrison C (2016) DNA vaccines against Zika virus speed into clinical trials. Nat Rev Drug Discov 15(8):521–522
Abbink, P., et al., Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science, 2016.
Zhao H et al (2016) Structural basis of Zika virus-specific antibody protection. Cell 166(4):1016–1027
Dai L et al (2016) Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 19(5):696–704
Acknowledgements
AHK is grateful for grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2014/16711-6) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (308608/2014-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Walter, L.T., Higa, G.S.V., Ikebara, J.M. et al. Evaluation of Possible Consequences of Zika Virus Infection in the Developing Nervous System. Mol Neurobiol 55, 1620–1629 (2018). https://doi.org/10.1007/s12035-017-0442-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0442-5