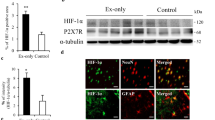
Abstract
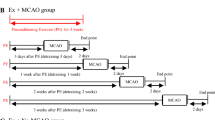
Subarachnoid hemorrhage (SAH) is a catastrophic form of stroke responsible for significant morbidity and mortality. Oxidative stress, inflammation, and neuronal apoptosis are important in the pathogenesis of early brain injury (EBI) following SAH. Preconditioning exercise confers neuroprotective effects, mitigating EBI; however, the basis for such protection is unknown. We investigated the effects of preconditioning exercise on brain damage and sensorimotor function after SAH. Male rats were assigned to either a sham-operated (Sham) group, exercise (Ex) group, or no-exercise (No-Ex) group. After a 3-week exercise program, they underwent SAH by endovascular perforation. Consciousness level, neurological score, and sensorimotor function were studied. The expression of nuclear factor erythroid 2 p45-related factor 2 (Nrf2), heme oxygenase 1 (HO-1), 4-hydroxynonenal (4HNE), nitrotyrosine (NT), ionized calcium-binding adaptor molecule 1 (Iba1), tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), interleukin 1β (IL-1β), 14–3-3γ, p-β-catenin Ser37, Bax, and caspase-3 were evaluated by immunohistochemistry or western blotting. The terminal deoxynucleotidyl transferase-mediated biotinylated dUTP nick end labeling (TUNEL) assay was also performed. After SAH, the Ex group had significantly reduced neurological deficits, sensorimotor dysfunction, and consciousness disorder compared with the No-Ex group. Nrf2, HO-1, and 14–3-3γ were significantly higher in the Ex group, while 4HNE, NT, Iba1, TNF-α, IL-6, IL-1β, Bax, caspase-3, and TUNEL-positive cells were significantly lower. Our findings suggest that preconditioning exercise ameliorates EBI after SAH. The expression of 4HNE and NT was reduced by Nrf2/HO-1 pathway activation; additionally, both oxidative stress and inflammation were reduced. Furthermore, preconditioning exercise reduced apoptosis, likely via the 14–3-3γ/p-β-catenin Ser37/Bax/caspase-3 pathway.








Similar content being viewed by others
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Schertz M, Mehdaoui H, Hamlat A, Piotin M, Banydeen R, Mejdoubi M (2016) Incidence and mortality of spontaneous subarachnoid hemorrhage in Martinique. PLoS ONE 11:e0155945. https://doi.org/10.1371/journal.pone.0155945
Gris T, Laplante P, Thebault P, Cayrol R, Najjar A, Joannette-Pilon B, Brillant-Marquis F, Magro E et al (2019) Innate immunity activation in the early brain injury period following subarachnoid hemorrhage. J Neuroinflammation 16:253. https://doi.org/10.1186/s12974-019-1629-7
Bederson JB, Connolly ES, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE Jr, Harbaugh RE et al (2009) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council, American Heart Association. Stroke 40:994–1025. https://doi.org/10.1161/STROKEAHA.108.191395
Zolnourian A, Galea I, Bulters D (2019) Neuroprotective role of the Nrf2 pathway in subarachnoid haemorrhage and its therapeutic potential. Oxid Med Cell Longev 2019:6218239. https://doi.org/10.1155/2019/6218239
Velat GJ, Kimball MM, Mocco JD, Hoh BL (2011) Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurg 76:446–454. https://doi.org/10.1016/j.wneu.2011.02.030
Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J et al (2014) Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol 115:64–91. https://doi.org/10.1016/j.pneurobio.2013.09.002
Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH (2013) Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 4:432–446. https://doi.org/10.1007/s12975-013-0257-2
Ostrowski RP, Colohan AR, Zhang JH (2006) Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res 28:399–414. https://doi.org/10.1179/016164106X115008
Zhu L, Ye T, Tang Q, Wang Y, Wu X, Li H, Jiang Y (2016) Exercise preconditioning regulates the toll-like receptor 4/nuclear factor-κB signaling pathway and reduces cerebral ischemia/reperfusion inflammatory injury: a study in rats. J Stroke Cerebrovasc Dis 25:2770–2779. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.07.033
Zhao X, Wen L, Dong M, Lu X (2016) Sulforaphane activates the cerebral vascular Nrf2–ARE pathway and suppresses inflammation to attenuate cerebral vasospasm in rat with subarachnoid hemorrhage. Brain Res 1653:1–7. https://doi.org/10.1016/j.brainres.2016.09.035
Islam MR, Young MF, Wrann CD (2017) Neuroprotective potential of exercise preconditioning in stroke. Cond Med 1:27–34
Sakakima H (2019) Endogenous neuroprotective potential due to preconditioning exercise in stroke. Phys Ther Res 22:45–52. https://doi.org/10.1298/ptr.r0006
Otsuka S, Sakakima H, Terashi T, Takada S, Nakanishi K, Kikuchi K (2019) Preconditioning exercise reduces brain damage and neuronal apoptosis through enhanced endogenous 14–3-3γ after focal brain ischemia in rats. Brain Struct Funct 224:727–738. https://doi.org/10.1007/s00429-018-1800-4
Rist PM, Capistrant BD, Mayeda ER, Liu SY, Glymour MM (2017) Physical activity, but not body mass index, predicts less disability before and after stroke. Neurology 88:1718–1726. https://doi.org/10.1212/WNL.0000000000003888
Baird L, Dinkova-Kostova AT (2011) The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol 85:241–272. https://doi.org/10.1007/s00204-011-0674-5
Kansanen E, Kuosmanen SM, Leinonen H, Levonenn AL (2013) The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol 1:45–49. https://doi.org/10.1016/j.redox.2012.10.001
de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J (2008) Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med 45:1375–1383. https://doi.org/10.1016/j.freeradbiomed.2008.09.001
Steel R, Cowan J, Payerne E, O’Connell MA, Searcey M (2012) Anti-inflammatory effect of a cell-penetrating peptide targeting the Nrf2/Keap1 interaction. ACS Med Chem Lett 3:407–410. https://doi.org/10.1021/ml300041g
Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G et al (2006) Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxidants Redox Signal 8:1975–1986. https://doi.org/10.1089/ars.2006.8.1975
Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D (2009) Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 110:469–485. https://doi.org/10.1111/j.1471-4159.2009.06160.x
Hascalovici JR, Song W, Liberman A, Vaya J, Khatib S, Holcroft C, Laferla F, Schipper HM (2014) Neural HO-1/sterol interactions in vivo: implications for Alzheimer’s disease. Neuroscience 280:40–49. https://doi.org/10.1016/j.neuroscience.2014.09.001
Shi Y, Sun X, Sun Y, Hou L, Yao M, Lian K, Li J, Lu X et al (2016) Elevation of cortical C26:0 due to the decline of peroxisomal β-oxidation potentiates amyloid β generation and spatial memory deficits via oxidative stress in diabetic rats. Neuroscience 315:125–135. https://doi.org/10.1016/j.neuroscience.2015.11.067
Yang Z, Weian C, Susu H, Hanmin W (2016) Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur J Pharmacol 771:145–151. https://doi.org/10.1016/j.ejphar.2015.12.003
Wang Z, Guo S, Wang J, Shen Y, Zhang J, Wu Q (2017) Nrf2/HO-1 mediates the neuroprotective effect of mangiferin on early brain injury after subarachnoid hemorrhage by attenuating mitochondria-related apoptosis and neuroinflammation. Sci Rep 7:11883. https://doi.org/10.1038/s41598-017-12160-6
Aguiar AS, Duzzioni M, Remor AP, Tristão FS, Matheus FC, Raisman-Vozari R, Latini A, Prediger RD (2016) Moderate-intensity physical exercise protects against experimental 6-hydroxydopamine-induced hemiparkinsonism through Nrf2-antioxidant response element pathway. Neurochem Res 41:64–72. https://doi.org/10.1007/s11064-015-1709-8
Tutakhail A, Nazary QA, Lebsir D, Kerdine-Romer S, Coudore F (2018) Induction of brain Nrf2-HO-1 pathway and antinociception after different physical training paradigms in mice. Life Sci 209:149–156. https://doi.org/10.1016/j.lfs.2018.08.004
Uekawa K, Hasegawa Y, Ma M, Nakagawa T, Katayama T, Sueta D, Toyama K, Kataoka K et al (2014) Rosuvastatin ameliorates early brain injury after subarachnoid hemorrhage via suppression of superoxide formation and nuclear factor-kappa B activation in rats. J Stroke Cerebrovasc Dis 23:1429–1439. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.12.004
Epah J, Pálfi K, Dienst FL, Malacarne PF, Bremer R, Salamon M, Kumar S, Jo H et al (2018) 3D imaging and quantitative analysis of vascular networks: a comparison of ultramicroscopy and micro-computed tomography. Theranostics 8:2117–2133. https://doi.org/10.7150/thno.22610
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167:327–334. https://doi.org/10.1016/j.jneumeth.2007.08.004
Isobe A, Kawaguchi M (2019) Relationship between motor function and ultrasonic vocalizations induced by maternal separation in rat pups. J Vet Med Sci 81:287–293. https://doi.org/10.1292/jvms.18-0604
MacIver MB, Bland BH, Hutt A (2014) Chaos analysis of EEG during isoflurane-induced loss of righting in rats. Front Syst Neurosci 8:203. https://doi.org/10.3389/fnsys.2014.00203
Grin’kina NM, Li Y, Haber M, Sangobowale M, Nikulina E, Le’Pre C, El Sehamy AM, Dugue R et al (2016) Righting reflex predicts long-term histological and behavioral outcomes in a closed head model of traumatic brain injury. PLoS ONE 11:e0161053. https://doi.org/10.1371/journal.pone.0161053
Chen G, Fang Q, Zhang J, Zhou D, Wang Z (2011) Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J Neurosci Res 89:515–523. https://doi.org/10.1002/jnr.22577
Shi SS, Zhang HB, Wang CH, Yang WZ, Liang RS, Chen Y, Tu XK (2015) Propofol attenuates early brain injury after subarachnoid hemorrhage in rats. J Mol Neurosci 57:538–545. https://doi.org/10.1007/s12031-015-0634-2
Otsuka S, Sakakima H, Sumizono M, Takada S, Terashi T, Yoshida Y (2016) The neuroprotective effects of preconditioning exercise on brain damage and neurotrophic factors after focal brain ischemia in rats. Behav Brain Res 303:9–18. https://doi.org/10.1016/j.bbr.2016.01.049
Ding YH, Li J, Yao WX, Rafols JA, Clark JC, Ding Y (2006) Exercise preconditioning upregulates cerebral integrins and enhances cerebrovascular integrity in ischemic rats. Acta Neuropathol 112:74–84. https://doi.org/10.1007/s00401-006-0076-6
Kalogeraki E, Pielecka-Fortuna J, Hüppe JM, Löwel S (2016) Physical exercise preserves adult visual plasticity in mice and restores it after a stroke in the somatosensory cortex. Front Aging Neurosci 8:212. https://doi.org/10.3389/fnagi.2016.00212
Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y (2008) Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol 115:289–296. https://doi.org/10.1007/s00401-008-0340-z
Wang RY, Yang YR, Yu SM (2001) Protective effects of treadmill training on infarction in rats. Brain Res 922:140–143. https://doi.org/10.1016/S0006-8993(01)03154-7
Liebelt B, Papapetrou P, Ali A, Guo M, Ji X, Peng C, Rogers R, Curry A et al (2010) Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience 166:1091–1100. https://doi.org/10.1016/j.neuroscience.2009.12.067
Guo M, Lin V, Davis W, Huang T, Carranza A, Sprague S, Reyes R, Jimenez D et al (2008) Preischemic induction of TNF-α by physical exercise reduces blood-brain barrier dysfunction in stroke. J Cereb Blood Flow Metab 28:1422–1430. https://doi.org/10.1038/jcbfm.2008.29
Dornbos D 3rd, Zwagerman N, Guo M, Ding JY, Peng C, Esmail F, Sikharam C, Geng X et al (2013) Preischemic exercise reduces brain damage by ameliorating metabolic disorder in ischemia/reperfusion injury. J Neurosci Res 91:818–827. https://doi.org/10.1002/jnr.23203
Feng R, Zhang M, Wang X, Li WB, Ren SQ, Zhang F (2014) Pre-ischemic exercise alleviates oxidative damage following ischemic stroke in rats. Exp Ther Med 8:1325–1329. https://doi.org/10.3892/etm.2014.1874
Wang Z, Chen G, Zhu WW, Zhou D (2010) Activation of nuclear factor-erythroid 2-related factor 2 (Nrf2) in the basilar artery after subarachnoid hemorrhage in rats. Ann Clin Lab Sci 40:233–239
Li T, Wang H, Ding Y, Zhou M, Zhou X, Zhang X, Ding K, He J et al (2014) Genetic elimination of Nrf2 aggravates secondary complications except for vasospasm after experimental subarachnoid hemorrhage in mice. Brain Res 1558:90–99. https://doi.org/10.1016/j.brainres.2014.02.036
Kwon SH, Ma SX, Hwang JY, Lee SY, Jang CG (2015) Involvement of the Nrf2/HO-1 signaling pathway in sulfuretin-induced protection against amyloid beta25-35 neurotoxicity. Neuroscience 304:14–28. https://doi.org/10.1016/j.neuroscience.2015.07.030
Crilly MJ, Tryon LD, Erlich AT, Hood DA (2016) The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J Appl Physiol 121:730–740. https://doi.org/10.1152/japplphysiol.00042.2016
Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L et al (2012) Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med 52:366–376. https://doi.org/10.1016/j.freeradbiomed.2011.10.440
Abreu CC, Cardozo LFMF, Stockler-Pinto MB, Esgalhado M, Barboza JE, Frauches R, Mafra D (2017) Does resistance exercise performed during dialysis modulate Nrf2 and NF-κB in patients with chronic kidney disease? Life Sci 188:192–197. https://doi.org/10.1016/j.lfs.2017.09.007
Tsou YH, Shih CT, Ching CH, Huang JY, Jen CJ, Yu L, Kuo YM, Wu FS et al (2015) Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP+ toxicity. Exp Neurol 263:50–62. https://doi.org/10.1016/j.expneurol.2014.09.021
Breitzig M, Bhimineni C, Lockey R, Kolliputi N (2016) 4-Hydroxy-2-nonenal: a critical target in oxidative stress? Am J Physiol Cell Physiol 311:C537–C543. https://doi.org/10.1152/ajpcell.00101.2016
Siuta M, Zuckerman SL, Mocco J (2013) Nitric oxide in cerebral vasospasm: theories, measurement, and treatment. Neurol Res Int 2013:972417. https://doi.org/10.1155/2013/972417
Kuhn DM, Sakowski SA, Sadidi M, Geddes TJ (2004) Nitrotyrosine as a marker for peroxynitrite-induced neurotoxicity: the beginning or the end of the end of dopamine neurons? J Neurochem 89:529–536. https://doi.org/10.1111/j.1471-4159.2004.02346.x
Darwish RS, Amiridze N, Aarabi B (2007) Nitrotyrosine as an oxidative stress marker: evidence for involvement in neurologic outcome in human traumatic brain injury. J Trauma 63:439–442. https://doi.org/10.1097/TA.0b013e318069178a
Zhang Y, Yang X, Ge X, Zhang F (2019) Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed Pharmacother 109:726–733. https://doi.org/10.1016/j.biopha.2018.10.161
Wu L, Su Z, Zha L, Zhu Z, Liu W, Sun Y, Yu P, Wang Y et al (2019) Tetramethylpyrazine nitrone reduces oxidative stress to alleviate cerebral vasospasm in experimental subarachnoid hemorrhage models. Neuromolecular Med 21:262–274. https://doi.org/10.1007/s12017-019-08543-9
Sulkowski S, Sulkowska M, Chyczewska E, Musiatowicz B (1992) Free alveolar cells in experimental pulmonary emphysema. I. Statistical analysis of cellular composition and lung morphometry. Pneumonol Alergol Pol 60:13–19
Chio CC, Lin HJ, Tian YF, Chen YC, Lin MT, Lin CH, Chang CP, Hsu CC (2017) Exercise attenuates neurological deficits by stimulating a critical HSP70/NF-ΚB/IL-6/synapsin I axis in traumatic brain injury rats. J Neuroinflammation 14:1–18. https://doi.org/10.1186/s12974-017-0867-9
Bhowmick S, D’Mello V, Caruso D, Abdul-Muneer PM (2019) Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J Mol Med 97:1627–1641. https://doi.org/10.1007/s00109-019-01851-4
Hamakawa M, Ishida A, Tamakoshi K, Shimada H, Nakashima H, Noguchi T, Toyokuni S, Ishida K (2013) Repeated short-term daily exercise ameliorates oxidative cerebral damage and the resultant motor dysfunction after transient ischemia in rats. J Clin Biochem Nutr 53:8–14. https://doi.org/10.3164/jcbn.12-72
Friedrich V, Flores R, Sehba FA (2012) Cell death starts early after subarachnoid hemorrhage. Neurosci Lett 512:6–11. https://doi.org/10.1016/j.neulet.2012.01.036
Wu LY, Enkhjargal B, Xie ZY, Travis ZD, Sun CM, Zhou KR, Zhang TY, Zhu QQ et al (2020) Recombinant OX40 attenuates neuronal apoptosis through OX40-OX40L/PI3K/AKT signaling pathway following subarachnoid hemorrhage in rats. Exp Neurol 326:113179. https://doi.org/10.1016/j.expneurol.2020.113179
Terashi T, Otsuka S, Takada S, Nakanishi K, Ueda K, Sumizono M, Kikuchi K, Sakakima H (2019) Neuroprotective effects of different frequency preconditioning exercise on neuronal apoptosis after focal brain ischemia in rats. Neurol Res 41:510–518. https://doi.org/10.1080/01616412.2019.1580458
Li DJ, Li YH, Yuan HB, Qu LF, Wang P (2017) The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 68:31–42. https://doi.org/10.1016/j.metabol.2016.12.003
Tu T, Yin S, Pang J, Zhang X, Zhang L, Zhang Y, Xie Y, Guo K et al (2021) Irisin contributes to neuroprotection by promoting mitochondrial biogenesis after experimental subarachnoid hemorrhage. Front Aging Neurosci 13:640215. https://doi.org/10.3389/fnagi.2021.640215
Kikuchi K, Setoyama K, Kawahara KI, Nagasato T, Terashi T, Ueda K, Nakanishi K, Otsuka S et al (2017) Edaravone, a synthetic free radical scavenger, enhances alteplase-mediated thrombolysis. Oxid Med Cell Longev 2017:6873281. https://doi.org/10.1155/2017/6873281
Acknowledgements
We thank Sushil Dawka, ELS, Andrea Baird, MD, and Bronwen Gardner, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Funding
This study was supported by grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant Nos. JP16K10746 and JP20K11640 to Kiyoshi Kikuchi and JP20K19312 to Shotaro Otsuka), General Insurance Association of Japan (to Kiyoshi Kikuchi), ZENKYOREN (National Mutual Insurance Federation of Agricultural Cooperatives) of Japan (to Kiyoshi Kikuchi), Mitsui Sumitomo Insurance Welfare Foundation of Japan (to Kiyoshi Kikuchi), Descente and Ishimoto Memorial Foundation for the Promotion of Sports Science (to Kiyoshi Kikuchi), and Taiju Life Social Welfare Foundation (to Kiyoshi Kikuchi).
Author information
Authors and Affiliations
Contributions
Shotaro Otsuka wrote the manuscript and performed the animal, histochemical, and biochemical studies. Kentaro Setoyama conducted the subarachnoid hemorrhage modeling in rats. Seiya Takada, Kazuki Nakanishi, Takuto Terashi, Kosuke Norimatsu, and Akira Tani performed the animal study and quantitative analysis. Harutoshi Sakakima conducted the histochemical and biochemical studies. Salunya Tancharoen conducted the animal study and wrote the manuscript. Ikuro Maruyama and Eiichiro Tanaka performed the literature review. Kiyoshi Kikuchi conducted the animal study, performed the literature review, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The experimental protocol was approved by the ethics board of the Institute of Experimental Animal Science of Kagoshima University.
Consent for Publication
Not applicable.
Consent to Participate
Not applicable.
Competing Interests
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Otsuka, S., Setoyama, K., Takada, S. et al. Preconditioning Exercise in Rats Attenuates Early Brain Injury Resulting from Subarachnoid Hemorrhage by Reducing Oxidative Stress, Inflammation, and Neuronal Apoptosis. Mol Neurobiol 58, 5602–5617 (2021). https://doi.org/10.1007/s12035-021-02506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02506-7