Abstract
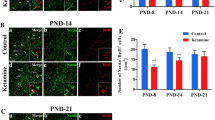
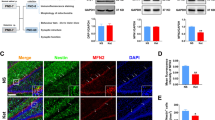
The Notch signaling pathway plays an important role in the regulation of neurogenesis. The objective of this study was to investigate whether the Notch signaling pathway was involved in the neurogenesis impairment and long-term neurocognitive dysfunction caused by neonatal exposure to ketamine. On postnatal day 7 (PND-7), male Sprague–Dawley (SD) rats were intraperitoneally injected with 40 mg/kg ketamine four consecutive times (40 mg/kg × 4) at 1-h intervals. Notch ligand Jagged1 (0.5 mg/kg) and lentivirus overexpressing the Notch1 intracellular domain (LV-NICD1) were microinjected into the hippocampal dentate gyrus (DG) 1 h or 4 days before ketamine administration, respectively. The expression of Notch1 signaling pathway-related proteins was detected by Western blotting 24 h after ketamine administration. The proliferation and differentiation of the neural stem cells (NSCs) in the hippocampal DG were evaluated by double immunofluorescence staining 24 h after treatment. Moreover, changes in hippocampus-dependent spatial memory of 2-month-old rats were investigated with the Morris water maze test. Ketamine anesthesia in neonatal rats decreased the expression levels of Jagged1, Notch1, NICD1, and hairy enhancer of split 1 (Hes1); inhibited the proliferation and astrocytic differentiation of NSCs; and promoted the differentiation of neurons. Neonatal exposure to ketamine caused deficits in hippocampus-dependent spatial reference memory tasks in 2-month-old rats. Microinjection of Jagged1 or LV-NICD1 reversed the inhibitory effect of ketamine on the expression of Notch1-related proteins in the hippocampal DG, attenuated the ketamine-mediated decrease in NSC proliferation and differentiation, and improved the cognitive function of 2-month-old rats after neonatal exposure to ketamine. These results suggest that neonatal exposure to ketamine in rats inhibits the proliferation and differentiation of hippocampal NSCs and impairs neurocognitive function in adulthood. The Notch1 signaling pathway may be involved in the impairment of hippocampus-dependent learning and memory during adulthood caused by neonatal exposure to ketamine. These findings contribute to further understanding the neurotoxicity induced by neonatal exposure to ketamine and the underlying mechanisms.









Similar content being viewed by others
Data availability
All data during this study are included in the supplementary information file.
The experimental protocol was approved by the Institutional Animal Care and Ethics Committee of Xuzhou Medical University.
References
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882
Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L (2011) Developmental stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology 115:282–293
Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Hu D, Voigt RG, Paule MG, Chelonis JJ, Flick RP (2018) Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) study. Anesthesiology 129:89–105
Cevik B, Tuncer M, Erkal KH, Sarica K (2018) Procedural sedation and analgesia for pediatric shock wave lithotripsy: a 10 year experience of single institution. Urolithiasis 46:363–367
Schmitz A, Weiss M, Kellenberger C, O’Gorman TR, Klaghofer R, Scheer I, Makki M, Sabandal C, Buehler PK (2018) Sedation for magnetic resonance imaging using propofol with or without ketamine at induction in pediatrics-a prospective randomized double-blinded study. Paediatr Anaesth 28:264–274
Bhatt M, Johnson DW, Chan J, Taljaard M, Barrowman N, Farion KJ, Ali S, Beno S, Dixon A, McTimoney CM, Dubrovsky AS, Sourial N, Roback MG (2017) Sedation Safety Study Group of Pediatric Emergency Research Canada (PERC): Risk factors for adverse events in emergency department procedural sedation for children. JAMA Pediatr 171:957–964
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283:70–74
Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W Jr, Wang C (2009) Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci 27:727–731
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W Jr, Wang C (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol and teratol 33:220–230
Pfenninger EG, Durieux ME, Himmelseher S (2002) Cognitive impairment after small-dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. Anesthesiology 96:357–366
Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schoeder DR, Weaver AL, Warner DO (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804
Bayer SA, Altman J, Russo RJ, Zhang X (1993) Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14:83–144
Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL (2013) Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 33:7368–7383
Doe CQ (2008) Neural stem cells: balancing self-renewal with differentiation. Development 135:1575–1587
Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, Alvarez-Buylla A (2015) Embryonic origin of postnatal neural stem cells. Cell 161:1644–1655
Hermann M, Reumann R, Schostak K, Kement D, Gelderblom M, Bernreuther C, Frischknecht R, Schipanski A, Marik S, Krasemann S, Sepulveda-Falla D, Schweizer M, Magnus T, Glatzel M, Galliciotti G (2020) Deficits in developmental neurogenesis and dendritic spine maturation in mice lacking the serine protease inhibitor neuroserpin. Mol Cell Neurosci 102:103420
Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11:339–350
Kang E, Berg DA, Furmanski O, Jackson WM, Ryu YK, Gray CD, Mintz CD (2017) Neurogenesis and developmental anesthetic neurotoxicity. Neurotoxicol Teratol 60:33–39
Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R (2009) Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 110:834–848
Fang F, Xue Z, Cang J (2012) Sevoflurane exposure in 7-day-old rats affects neurogenesis, neurodegeneration and neurocognitive function. Neurosci Bull 28:499–508
Dong C, Rovnaghi CR, Anand KJ (2012) Ketamine alters the neurogenesis of rat cortical neural stem progenitor cells. Crit Care Med 40:2407–2416
Bosnjak ZJ, Logan S, Liu Y, Bai X (2016) Recent insights into molecular mechanisms of propofol-induced developmental neurotoxicity: implications for the protective strategies. Anesth Analg 123:1286–1296
Huang H, Liu CM, Sun J, Hao T, Xu CM, Wang D, Wu YQ (2016) Ketamine affects the neurogenesis of the hippocampal dentate gyrus in 7-day-old. Neurotox Res 30:185–198
Zhao Z, Li B, Wu Y, Chen X, Guo Y, Shen Y, Huang H (2019) Ketamine affects the integration of developmentally generated granule neurons in the adult stage. BMC Neurosci 20(1):60
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signalling pathway: cell fate control and signalling pathway integration in development. Science 284:770–776
Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ (2000) Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101:499–510
Lee JE (1997) Basic helix-loop-helix genes in neural development. Curr Opin Neurobiol 7:13–20
Iso T, Kedes L, Hamamori Y (2003) HES and HERP families: multiple effectors of the Notch signalling pathway. J Cell Physiol 194:237–255
Imayoshi I, Isomura A, Harima Y, Kawaguchi K, Kori H, Miyachi H, Fujiwara T, Ishidate F, Kageyama R (2013) Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science 342:1203–1208
Andersen J, Urban N, Achimastou A, Ito A, Simic M, Ullom K, Martynoga B, Lebel M, Goritz C, Frisen J, Nakafuku M, Guillemot F (2014) A transcriptional mechanism integrating inputs from extracellular signalling pathway to activate hippocampal stem cells. Neuron 83:1085–1097
Shi Y, Li JJ, Chen CJ, Xia YW, Li YX, Zhang P, Xu Y, Li TY, Zhou WH, Song WH (2018) Ketamine modulates Zic5 expression via the Notch signalling pathway in neural crest induction. Front Mol Neurosci 7:9
Sun F, Mao X, Xie L, Ding M, Shao B, Jin K (2013) Notch1 signaling modulates neuronal progenitor activity in the subventricular zone in response to aging and focal ischemia. Aging Cell 12:978–987
Ashwell KWS, Paxinos G: Atlas of the developing rat nervous system. Elsevier 2008; San Diego
Paxinos G, Watson C: The rat brain in stereotaxic coordinates. Academic Press 1986; Sydney
Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW, Takagi MJ, Giribaldi G, Hartmann PL, Salvo I, Morton NS, von Ungern Sternberg BS, Locatelli BG, Wilton N, Lynn A, Thomas JJ, Polaner D, Bagshaw O, Szmuk P, Absalom AR, Frawley G, Berde C, Ormond GD, Marmor J, McCann ME (2016) consortium, G.A.S: Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS) an international multicentre, randomised controlled trial. Lancet 387:239–50
Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, Ing C, Park R, Radcliffe J, Hays SR, DiMaggio CJ, Cooper TJ, Rauh V, Maxwell LG, Youn A, McGowan FX (2016) Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 315:2312–2320
Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Hu D, Voigt RG, Paule MG et al (2018) Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) study. Anesthesiology 129:89–105
Andropoulos DB, Greene MF (2017) Anesthesia and developing brains-implications of the FDA warning. N Engl J Med 376:905–907
Belnoue L, Grosjean N, Ladeveze E, Abrous DN, Koehl M (2013) Prenatal stress inhibits hippocampal neurogenesis but spares olfactory bulb neurogenesis. PLoS ONE 8:e72972
Porzionato A, Macchi V, Zaramella P, Sarasin G, Grisafifi D, Dedja A, Chiandetti L, De Caro R (2013) Effects of postnatal hyperoxia exposure on the rat dentate gyrus and subventricular zone. Brain Struct Funct 220:229–247
Yamamura H, Yamamura A, Ko EA, Pohl NM, Smith KA, Zeifman A, Powell FL, Thistlethwaite PA, Yuan JX (2014) Activation of Notch signaling by short-term treatment with Jagged-1 enhances store-operated Ca(2+) entry in human pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 306:C871–C878
Zuo Q, Zhang C, Jin K, Jing J, Sun C, Ahmed MF, Song J, Zhang Y, Chen G, Li B (2018) NICD-mediated notch transduction regulates the different fate of chicken primordial germ cells and spermatogonial stem cells. Cell Biosci 8:40
Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C (2010) Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6:445–456
Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F (2016) Age-dependent niche signalling pathway from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 19:643–652
Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P (2007) Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. PNAS 104:20558–20563
Ohtsuka T, Sakamoto M, Guillemot F, Kageyama R (2001) Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J Biol Chem 276:30467–30474
Nakamura Y, Sakakibara S, Miyata T, Ogawa M, Shimazaki T, Weiss S, Kageyama R, Okano H (2000) The bHLH gene hes1 as a repressor of the neuronal commitment of CNS stem cells. J Neurosci 20:283–293
Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodríguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN (2007) Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol 5:e214
Wang L, Chopp M, Zhang RL, Zhang L, Letourneau Y, Feng YF, Jiang A, Morris DC, Zhang ZG (2009) The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience 158:1356–1363
Giachino C, Basak O, Lugert S, Knuckles P, Obernier K, Fiorelli R, Frank S, Raineteau O, Alvarez-Buylla A, Taylor V (2014) Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells 32:70–84
Altman J, Bayer SA (1990) Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol 301:365–381
Funding
This work was supported by the National Natural Science Foundation of China (81171013, 81901100), Jiangsu Province Special Program for Young Medical Talent (QNRC2016587), and the Natural Science Foundation of Jiangsu Province (BK20191464).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Yu-Qing Wu, He Huang, Chao Zhao, and Cheng-Hua Zhou; performed the experiments: He Huang, Chao Zhao, Qiang Liu, Yi-Man Sun, Chen Chen, and Hui Huang; data analysis and interpretation: Qian Hu, He Huang, and Yu-Qing Wu; manuscript preparation: He Huang and Yu-Qing Wu.
Corresponding authors
Ethics declarations
Consent to participate
Not applicable.
Consent for Publication
Yes.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, H., Zhao, C., Hu, Q. et al. Neonatal Anesthesia by Ketamine in Neonatal Rats Inhibits the Proliferation and Differentiation of Hippocampal Neural Stem Cells and Decreases Neurocognitive Function in Adulthood via Inhibition of the Notch1 Signaling Pathway. Mol Neurobiol 58, 6272–6289 (2021). https://doi.org/10.1007/s12035-021-02550-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02550-3