Abstract
Wolbachia, a bacterium that infects insect populations, has been examined extensively in Drosophila populations and, in recent years, has garnered significant attention for its potential to reduce the spread of dengue in the Aedes mosquito population. Similar applications to Anopheles mosquitoes for the reduction of malaria have not been as thoroughly studied, as Anopheles were previously thought to be devoid of Wolbachia infection. The recent discovery, however, of Wolbachia in two separate wild Anopheles populations suggests further study is needed. We develop and analyze an ordinary differential equation model of Wolbachia infection in Anopheles mosquitoes, which demonstrate different reproductive phenotypes than Aedes mosquitoes when infected with Wolbachia. In particular, they do not show the hallmark cytoplasmic incompatibility phenotype—absence of viable offspring when infected males mate with uninfected females—or other standard sex-biasing phenotypes. Instead, evidence of increased speed of gonotrophic cycles by Wolbachia-infected females has been reported. We show that the ability for Wolbachia to invade for a basic reproductive number less than 1 (Rpop < 1), found in other models, is significantly diminished here. However, the invasion threshold below Rpop < 1 can be partially recovered with the increased speed of laying eggs, as incorporated through gonotrophic cycles. Our results highlight the need for further experimental and theoretical work if Wolbachia is to be considered as a form of malaria control.










Similar content being viewed by others
References
Baldini F, Segata N, Pompon J, Marcenac P, Shaw WR, Dabiré RK, Diabaté A, Levashina EA, Catteruccia F (2014) Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat Commun 5:3985
Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, Xu Y, Dimopoulos G, Xi Z (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium Infection. Science 340 (6133):748–751. https://doi.org/10.1126/science.1236192
Caragata EP, Dutra HL, Moreira LA (2016) Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends in Parasitology 32(3):207–218. https://doi.org/10.1016/j.pt.2015.10.011. Special Issue: Vectors
Caspari E, Watson G (1959) On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution 13(4):568–570
Childs LM, Cai FY, Kakani EG, Mitchell SN, Paton D, Gabrieli P, Buckee CO, Catteruccia F (2016) Disrupting mosquito reproduction and parasite development for malaria control. PLoS Pathogens 12 (12):e1006,060
Christiansen-Jucht C, CY S, Basáñez M, Parham P (2015) Modelling Anopheles gambiae s.s. population dynamics with temperature- and age-dependent survival. International Journal on Environmental Research and Public Health 12:5975–6005
Christiansen-Jucht CD, Parham PE, Saddler A, Koella JC, Basáñez MG (2015) Larval and adult environmental temperatures influence the adult reproductive traits of Anopheles gambiae ss. Parasites & Vectors 8 (1):456
Clements A, Paterson G (1981) The analysis of mortality and survival rates in wild populations of mosquitoes. Journal of applied ecology, pp 373–399
Crain PR, Mains JW, Suh E, Huang Y, Crowley PH, Dobson SL (2011) Wolbachia infections that reduce immature insect survival: predicted impacts on population replacement. BMC Evol Biol 11(1):290. https://doi.org/10.1186/1471-2148-11-290
Dawes EJ, Churcher TS, Zhuang S, Sinden RE, Basáñez MG (2009) Anopheles mortality is both age-and plasmodium-density dependent: implications for malaria transmission. Malar J 8(1):228
Dieter KL, Huestis DL, Lehmann T (2012) The effects of oviposition-site deprivation on Anopheles gambiae reproduction. Parasites & Vectors 5(1):235
Dobson SL, Fox CW, Jiggins FM (2002) The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proceedings of the Royal Society of London B:, Biological Sciences 269(1490):437–445. https://doi.org/10.1098/rspb.2001.1876
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(1):29–48. https://doi.org/10.1016/S0025-5564(02)00108-6
Eikenberry SE, Gumel AB (2018) Mathematical modeling of climate change and malaria transmission dynamics: a historical review. Journal of Mathematical Biology. https://doi.org/10.1007/s00285-018-1229-7
Farkas JZ, Hinow P (2010) Structured and unstructured continuous models for Wolbachia infections. Bull Math Biol 72(8):2067–2088. https://doi.org/10.1007/s11538-010-9528-1
Gillies M, Wernsdorfer WH, Mcgregor I (1988) Malaria: principles and practice of malariology
Gomes FM, Hixson BL, Tyner MDW, Ramirez JL, Canepa GE, Alves e Silva TL, Molina-Cruz A, Keita M, Kane F, Traoré B, Sogoba N, Barillas-Mury C (2017) Effect of naturally occurring Wolbachia in Anopheles gambiae s.l. mosquitoes from Mali on Plasmodium falciparum malaria transmission. In: Proceedings of the National Academy of Sciences. https://doi.org/10.1073/pnas.1716181114
Hamm CA, Begun DJ, Vo A, Smith CC, Saelao P, Shaver AO, Jaenike J, Turelli M (2014) Wolbachia do not live by reproductive manipulation alone: infection polymorphism in Drosophila suzukii and D. subpulchrella. Mol Ecol 23(19):4871–4885
Hancock PA, Sinkins SP, Godfray HCJ (2011) Population dynamic models of the spread of Wolbachia. The American Naturalist 177(3):323–333. https://doi.org/10.1086/658121. PMID: 21460541
Hoffmann A, Montgomery B, Popovici J, Iturbe-Ormaetxe I, Johnson P, Muzzi F, Greenfield M, Durkan M, Leong Y, Dong Y et al (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476(7361):454
Huang M, Tang M, Yu J (2015) Wolbachia infection dynamics by reaction-diffusion equations. Sci China Math 58(1):77–96. https://doi.org/10.1007/s11425-014-4934-8
Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL (2011) Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog 7(5):1–8. https://doi.org/10.1371/journal.ppat.1002043
Hughes H, Britton NF (2013) Modelling the use of Wolbachia to control dengue fever transmission. Bull Math Biol 75(5):796–818. https://doi.org/10.1007/s11538-013-9835-4
Jansen VA, Turelli M, Godfray HCJ (2008) Stochastic spread of Wolbachia. Proceedings of the Royal Society of London B:, Biological Sciences 275(1652):2769–2776. https://doi.org/10.1098/rspb.2008.0914
Kambris Z, Blagborough AM, Pinto SB, Blagrove MSC, Godfray HCJ, Sinden RE, Sinkins SP (2010) Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog 6(10):1–9. https://doi.org/10.1371/journal.ppat.1001143
Keeling M, Jiggins F, Read J (2003) The invasion and coexistence of competing Wolbachia strains. Heredity 91:382–388
Kittayapong P, Baisley KJ, Baimai V, O’Neill SL (2000) Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (diptera: Culicidae). J Med Entomol 37(3):340–345. https://doi.org/10.1093/jmedent/37.3.340
Koiller J, Da Silva M, Souza M, Codeço C, Iggidr A, Sallet G (2014) Aedes, Wolbachia and Dengue. Research Report RR-8462, inria nancy - grand est, publisher=Villers-lès-Nancy, address=France)
Kriesner P, Conner WR, Weeks AR, Turelli M, Hoffmann AA (2016) Persistence of a Wolbachia infection frequency cline in Drosophila melanogaster and the possible role of reproductive dormancy. Evolution 70 (5):979–997
Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR (2013) Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathogens 9(9):e1003,607
Li J, Blakeley D et al (2011) The failure of R0. Computational and Mathematical Methods in Medicine 2011
Mala AO, Irungu LW, Mitaki EK, Shililu JI, Mbogo CM, Njagi JK, Githure JI (2014) Gonotrophic cycle duration, fecundity and parity of Anopheles gambiae complex mosquitoes during an extended period of dry weather in a semi arid area in Baringo County, Kenya. Int J Mosq Res 1(2):28–34
McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, O’Neill SL (2009) Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323(5910):141–144
McMeniman CJ, O’Neill SL (2010) A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl Trop Dis 4(7):e748
Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O’Neill SL (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139(7):1268–1278. https://doi.org/10.1016/j.cell.2009.11.042
Murdock C, Blanford S, Hughes G, Rasgon J, Thomas M (2014) Temperature alter Plasmodium blocking by Wolbachia. Sci Rep 4:3932
Ndii M, Allingham D, Hickson R, Glass K (2016) The effect of Wolbachia on dengue dynamics in the presence of two serotypes of dengue: symmetric and asymmetric epidemiological characteristics. Epidemiol Infect 144(13):2874–2882
Ndii MZ, Hickson RI, Mercer GN (2012) Modelling the introduction of Wolbachia into Aedes aegypti mosquitoes to reduce dengue transmission. The ANZIAM J 53(3):213–227
O’Neill SL, Hoffman AA, Werren JH (1997) Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press
Paton DG, Childs LM, Itoe MA, Holmdahl IE, Buckee CO, Catteruccia F (2019) Exposing anopheles mosquitoes to antimalarials blocks plasmodium parasite transmission. Nature 567(7747):239
Qu Z, Xue L, Hyman JM (2018) Modeling the transmission of Wolbachia in mosquitoes for controlling mosquito-borne diseases. SIAM J Appl Math 78(2):826–852
Rasgon JL, Ren X, Petridis M (2006) Can Anopheles gambiae be infected with Wolbachia pipientis? insights from an in vitro system. Appl Environ Microbiol 72(12):7718–7722
Schofield P (2002) Spatially explicit models of Turelli-Hoffmann Wolbachia invasive wave fronts. J Theor Biol 215(1):121–131. https://doi.org/10.1006/jtbi.2001.2493
Schraiber JG, Kaczmarczyk AN, Kwok R, Park M, Silverstein R, Rutaganira FU, Aggarwal T, Schwemmer MA, Hom CL, Grosberg RK et al (2012) Constraints on the use of lifespan-shortening Wolbachia to control dengue fever. J Theor Biol 297:26–32
Shaw WR, Marcenac P, Childs LM, Buckee CO, Baldini F, Sawadogo SP, Dabiré RK, Diabaté A, Catteruccia F (2016) Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat Commun 7(11):772
Silva JBL, Alves DM, Bottino-Rojas V, Pereira TN, Sorgine MHF, Caragata EP, Moreira LA (2017) Wolbachia and dengue virus infection in the mosquito Aedes fluviatilis (diptera: Culicidae). PloS One 12 (7):e0181,678
Souto-Maior C, Lopes JS, Gjini E, Struchiner CJ, Teixeira LM, Gomes MG (2015) Heterogeneity in symbiotic effects facilitates Wolbachia establishment in insect populations. Theoretical Ecology 8(1):53–65. https://doi.org/10.1007/s12080-014-0235-7
Stouthamer R, Breeuwer JAJ, Luck RF, Werren JH (1993) Molecular identification of microorganisms associated with parthenogenesis. Nature 361:66–68
Styer LM, Carey JR, Wang JL, Scott TW (2007) Mosquitoes do senesce: departure from the paradigm of constant mortality. Am J Trop Med Hyg 76(1):111–117
Turelli M (2010) Cytoplasmic incompatibility in populations with overlapping generations. Evolution:, International Journal of Organic Evolution 64(1):232–241
Turelli M, Hoffmann AA (1991) Rapid spread of an inherited incompatibility factor in california Drosophila. Nature 353(6343):440
Walker T, Johnson P, Moreira L, Iturbe-Ormaetxe I, Frentiu F, McMeniman C, Leong Y, Dong Y, Axford J, Kriesner P et al (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476(7361):450
Walker T, Moreira LA (2011) Can Wolbachia be used to control malaria? Memórias do Instituto Oswaldo Cruz 106:212–217
Werren JH (1997) Biology of Wolbachia. Annu Rev Entomol 42(1):587–609. https://doi.org/10.1146/annurev.ento.42.1.587. PMID: 15012323
White MT, Griffin JT, Churcher TS, Ferguson NM, Basáñez MG, Ghani AC (2011) Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasites & Vectors 4(1):153
Xue L, Manore CA, Thongsripong P, Hyman JM (2017) Two-sex mosquito model for the persistence of Wolbachia. J Biol Dyn 11(sup1):216–237
Acknowledgments
We also thank two anonymous reviewers for their insightful comments on an early draft of this manuscript.
Funding
LMC received support from Simons Foundation: Collaboration Grant for Mathematicians Award 524390.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Analysis of mosquito population lacking Wolbachia infection
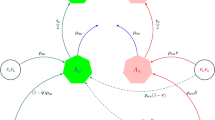
The system of equations for mosquito population dynamics where A is the aquatic population, M is the adult males, and Gj are adult females in gonotrophic cycle j is:
where ϕ is the per capita egg laying rate of females, Ka is the carrying capacity of aquatic mosquitoes, η is the development rate of aquatic mosquitoes, μi is the mortality rate of females in the i th gonotrophic cycle, m (f ) is the fraction of eggs that are male (female), and σ is the transition rate of adult females between gonotrophic cycles.
Epidemiologically and mathematically well-posed. The model for mosquito population dynamics is epidemiologically and mathematically well-posed. To show this, we demonstrate that the region:
is invariant under the flow from system (11) with \(\mu _{G_{j}}=\mu _{g}\) for all j, assuming that the initial conditions lie in the domain \(\mathcal {D}\).
In order to show this, we note that the right-hand sides of all Eqs. 11 have continuous partial derivatives in the specified domain. Along all the edges of the domain, the time derivatives lead the solution back into the invariant domain. We begin by examining when each of the variables is at its minimum:
Furthermore, when each variable is at their maximum we see:
We expect a similar result when relaxing the assumption that all female adult mortality rates are identical with a bit more complicated formula for the maximum of the adult female population.
Equilibria and stability. Assuming mortality is constant in the adult stage, i.e., \(\mu _{G_{i}}=\mu _{g}\), the extinction equilibrium given by:
is locally asymptotically stable when Rpop < 1, with:
The persistent equilibrium given by:
is locally asymptotically stable when Rpop > 1.
To show this, we consider the Jacobian matrix of system 11, which is:
with − (σ + μg) along the diagonal and σ along the subdiagonal in the part of the matrix that is suppressed. At the extinction equilibrium, this becomes
with − (σ + μg) along the diagonal and σ along the subdiagonal. The eigenvalues of this matrix are:
When Rpop < 1, then all eigenvalues are negative and the extinction equilibrium is locally asymptotically stable.
The Jacobian matrix at the persistent equilibrium is:
with − (σ + μg) along the diagonal and σ along the subdiagonal. The eigenvalues of this matrix are:
When Rpop > 1, then all eigenvalues are negative and the persistent equilibrium is locally asymptotically stable.
Appendix 2: Next-generation method matrices
In order to reduce the notational complexity, we assume two gonotrophic cycles for each type of adult female, i.e., Nu = 2 and Nw = 2, and that adult female mortality is only dependent on infection status (\(\mu _{G_{i}}=\mu _{g}\) and \(\mu _{W_{i}}=\mu _{w}\)).
Thus, the Jacobian of the matrix of new infections \(\mathcal {F}\) is:
Here, \(A_{u}^{*}\) refers to the uninfected aquatic population at the DFE.
The Jacobian of the matrix of transitions \(\mathcal {V}\) is:
Appendix 3: Comparison with other Wolbachia-infected mosquito models
In similarly formed models of Wolbachia-infected mosquitoes including both male and female adult mosquitoes (Qu et al. 2018; Xue et al. 2017), the authors made simplifying assumptions on the aquatic and male compartments that we do not include in this paper. They assume identical male mortality (\(\mu _{M_{u}}=\mu _{M_{w}}\)), identical aquatic mortality (\(\mu _{A_{u}}=\mu _{A_{w}}\)), and identical aquatic maturation (ηu = ηw) between uninfected and infected mosquitoes. In the following, we discuss how our analysis in “Endemic equilibrium” compares with their results.
Under perfect maternal transmission (q = 1), the authors found \(\left (\frac {1}{R_{\text {pop}}}-1\right )\) for what we call z, which determines the endemic equilibria. Under their parameter simplifications, then our α = 1, in which case we would also recover \(z=\left (\frac {1}{R_{\text {pop}}}-1\right )\).
In the absence of restrictions on maternal transmission 0 ≤ q ≤ 1, the authors found:
for what we call z. Under their parameter simplifications, in addition to our α = 1 and β = 1, then pwu = 0 as they always work in the setting of full cytoplasmic incompatibility. Using their parameter simplifications, we would recover:
Noticing that they also assume that q + p = 1, our result would be identical to their solution.
Our formula for the basic reproduction number Rpop is similar to that found by Qu et al. (2018) and Xue et al. (2017), \(R_{\text {pop}} = \frac {R_{\text {pop}}^{w}}{R_{\text {pop}}^{u}}\). If we assume the complete cytoplasmic incompatibility, i.e., pwu = 0 and their other parameter simplifications, then we recoup the same result.
Our model, despite similarity to previous work, retains the flexibility to allow for differences between the uninfected and infected populations in the aquatic and male stages as well as including variable levels of cytoplasmic incompatibility. In addition, we include a variable number of gonotrophic cycles for adult females and variable mortality across adult female stages.
Rights and permissions
About this article
Cite this article
Childs, L.M., Hughes, R. & Blackwood, J.C. The role of increased gonotrophic cycles in the establishment of Wolbachia in Anopheles populations. Theor Ecol 13, 349–369 (2020). https://doi.org/10.1007/s12080-020-00457-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-020-00457-8