Abstract
Soon after the discovery that viruses cause human disease, started the idea of using viruses to treat cancer. After the initial indiscriminate use, crude preparations of each novel virus in the early twentieth century, a second wave of virotherapy blossomed in the 60s with purified and selected viruses. Responses were rare and short-lived. Immune rejection of the oncolytic viruses was identified as the major problem and virotherapy was abandoned. During the past two decades virotherapy has re-emerged with engineered viruses, with a trend towards using them as tumor-debulking immunostimulatory agents combined with radio or chemotherapy. Currently, oncolytic Reovirus, Herpes, and Vaccinia virus are in late phase clinical trials. Despite the renewed hope, efficacy will require improving systemic tumor targeting, overcoming stroma barriers for virus spread, and selectively stimulating immune responses against tumor antigens but not against the virus. Virotherapy history, viruses, considerations for clinical trials, and hurdles are briefly overviewed.
Similar content being viewed by others
Viruses as a cancer cause and solution: historical perspective
A virus that can kill tumor cells in culture or in animal models is known as oncolytic and its use in cancer treatment as virotherapy. In relation to cancer, viruses are certainly better known as the cause in 20 % of human cancers: Epstein Barr virus causes Burkitts lymphoma, HPV causes cervical carcinoma, Hepatitis B and C viruses cause hepatocellular carcinoma, and Kaposi sarcoma Herpes virus causes Kaposi sarcoma. In 1908 Ellerman and Bang demonstrated that a virus caused chicken leukemias (for a review see [1]) and in 1911 Peyton Rose demonstrated that a filterable agent (a virus) caused chicken sarcoma. Duran I Reyanls corroborated these results and insisted that the filterable agent was a virus. His sentence “viruses are in the cancer problem to stay” has been fulfilled. But viruses may also be in the cancer solution to stay. The same Duran I Reynals already appreciated this other side of the coin, the possibility to use viruses to treat diseases, and he pioneered the therapeutic use of bacteriophages to treat bacterial infections. In fact, with regard to cancer the description of the role of viruses as a solution to cancer is much earlier than their role as a problem. In the last years of the nineteenth century, simultaneous to the discovery of viruses and their role in infectious diseases, there were occasional observations that cancer could regress in patients suffering infectious diseases of known viral etiology [2, 3]. These tumor regressions were more common in leukemias and lymphomas of young patients, where the immune system was compromised. But the regressions were very rare, incomplete, and lasted a few days or months. In any case such observations inspired the first attempts to use viruses to treat cancer (virotherapy) [4]. However, virotherapy did not blossom until the development of cell culture techniques in the mid-twentieth century that allowed the propagation and purification of viruses and their characterization. In the decades that followed, many clinical trials were done with Hepatitis virus, West Nile virus (flavivirus), Mumps virus, Coxsackie virus, Herpesvirus, Vaccinia virus, Adenovirus, etc. Alice Moore initiated the use of rodent models to test and select the best viruses for clinical trials that she next performed in collaboration with oncologist Chester Southam. Despite this virus selection, the results of these trials mimicked the results obtained 50 years earlier and summarize the conclusions that led to the abandonment of virotherapy in the 70s in favor of the emerging chemotherapy alternative: the virus punched a hole in the tumor until it was cleared by the immune system. Given the importance of the immune system in the poor clinical results and the observation that in immunocompetent animal models virotherapy could result in post-oncolytic antitumor immunity, the idea of using viruses as an immunotherapy strategy gained support, and tumor oncolysates of autologous or allogeneic tumor cells infected with viruses were used to vaccinate patients. Occasional and transient immune responses were reported but eventually even this ex vivo use of viruses was dismissed. It was not until the 90s, with the recombinant DNA technology available to modify the virus genomes, that a new wave of virotherapy initiated [5] with the hope that even more selective, potent, and immunoregulatory viruses could become a cancer therapy. In the 20th anniversary of this third wave, this hope still has not been fulfilled but it seems closer than ever.
Viruses and antitumor mechanisms
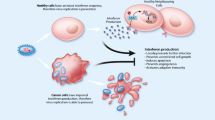
For its application to cancer therapy, an oncolytic virus is grown in permissive cell lines through multiples rounds of infection until a large amount of virus is harvested and purified in density gradients or chromatography columns. The virus in solution is characterized (identity and activity parameters) to be injected in patients via different routes (intratumorally, intracavitally, intravenously, or intra-arterial). For efficacy, the virus must reach tumor cells (tumor targeting), start the infectious lytic cycle to lyse such cells, and produce virus progeny able to spread through the tumor. Selectivity for cancer cells at the entry level or during the virus replication process is a trait of oncolytic viruses to allow therapy without harming normal tissues. The selective lysis of tumor cells is the first and most obvious mechanisms of virotherapy. In theory, in the absence of stroma barriers and antivirus immune responses (innate and adaptive), the virus would spread until the complete tumor mass is lysed. But clinical experience shows short-lived and partial antitumor responses with concomitant antivirus immunity indicating an efficient hurdle to the virus. In fact, clinical trials have not demonstrated that direct tumor cell lysis is an important mechanims to eliminate tumors [3]. However, the fact that immunity eliminates the virus from the infected tumor cells means that the strong immune suppressive environment that characterize tumors has been modified by the virus, hence raising the possibility of an antitumor immunotherapy mechanism [6]. The different tropism, structure, and life cycle of different oncolytic viruses have implications in these two mechanisms of virotherapy.
In general terms, RNA viruses are fast-growing viruses that replicate in the cytoplasm of infected cells (except for retroviruses). For RNA viruses, the interferon (IFN) pathway is central to their tumor-selective replication. Certain cells (fibroblasts and macrophages) secrete IFNs upon infection which bind IFN receptors on normal cells to induce and antiviral state characterized by protein translation inhibition. Tumor cells activate the Ras/MAPK and PI3K pathways to promote protein translation and are resistant to the IFN effects. Thus, viruses sensitive to IFN inhibition in normal cells will show a natural tropism to tumor cells. Viruses can be naturally sensitive to IFN inhibition (many RNA viruses) or can be modified genetically to be sensitive to IFN if the virus proteins responsible to counteract the IFN response are deleted. A caveat of highly IFN-sensitive oncolytic viruses is that most tumor cells are not completely IFN-resistant, as deduced from the fact that these viruses only grow efficiently in a very restricted number of cell lines in vitro (these cell lines are used for virus production purposes).
Reovirus (orthoreovirus) is a RNA virus without lipid envelope that replicates in the cytoplasm of the infected cell. The virion is a capsid double protein layer icosahedra of 70 nm of diameter that contains ten molecules of double-stranded RNA (segmented genome). Reovirus is common in the respiratory and gastrointestinal tract of humans (50–100 % seropositivity) but does not cause disease. The sensitivity to IFN inhibition in normal cells may explain its natural tropism for cancer cells, but currently neither the virus receptor levels (mainly the junction adhesion molecule 1, JAM1) nor markers of IFN or apoptosis correlates with the permissivity to the virus. Systemic (intravenous) administration of Reovirus (strain 3 or dearing, known as Reolysin) in combination with paclitaxel and carboplatin is in phase 3 trials for squamous carcinomas of the head and neck (see Table 1 for selected clinical trials). The low activity of Reovirus alone contrast with the clinical results obtained in combination with chemotherapy [7], suggesting that the sensitization to chemotherapy, likely via apoptosis, is the main mechanism of activity, even with low virus replication.
Newcastle Disease Virus (NDV) and Measles Virus (rubeola) are paramyxoviruses, enveloped viruses of one negative-strand RNA as a genome. Natural strains of NDV have been used in oncolysis due to their sensitivity to IFN. These strains of NDV are the main non-human viruses that still remain in vogue among many non-human viruses tested during the 50s and 60s. NDV causes a respiratory disease in poultry but no harm to exposed workers and the virus is considered safe for human application. Paramyxoviruses fuse cell membranes form syncytia which offers the advantage of an antibody-resistant cell-to-cell viral spread. However, they are halted by stroma as any other oncolytic virus [8]. In phase I clinical trials of intravenously administered NDV (strains PV701 and MTH-68/H) it has been observed that the initial virus infusions or very slow infusions reduce the toxicity to the virus and thus they are used as desensitization steps to increase the doses [9, 10]. With these optimized protocols partial responses are frequently observed and phase 2 trials are ongoing.
The vaccine strain Edmonston of Measles Virus is used as an oncolytic virus [11]. Measles infects cells through CD46, SLAM, and nectin 4 receptors, but the Edmonston strain prefers CD46 and nectin 4 which are overexpressed in tumors and thus confers tumor-selectivity additional to the gained by its IFN sensitivity. However, higher receptor selectivity has been achieved by modifiying the envelope H protein with ligands such single-chain antibodies. This strategy may help to bypass the effective virus neutralization expected in measles-vaccinated patients, in particular if used intravenously. Genetic modifications of NDV and Measles to improve its imaging, oncolytic potency, spread, and immunoregulatory properties are ongoing. For Measles, these engineered viruses have already reached phase 1 clinical trials [12].
With regard to DNA viruses, the most commonly used in virotherapy are Herpes Simples Virus 1, Vaccinia Virus, and Adenovirus. In general terms DNA viruses replicate in the nucleus of infected cells (except Vaccinia Virus) and have longer (slower) replication cycles than RNA viruses. However, transcription of virus genes with cellular RNA polymerase offers the possibility to control virus replication replacing virus promoters with tumor-specific promoters. This strategy has been used mainly with adenovirus, as the control of virus replication is not only under the E1a gene, but also with Herpes virus. The cytoplasmic replication of Vaccinia virus precludes the use of this transcriptional targeting strategy and Vaccinina virus tumor-selectivity is achieved by deleting genes that enhance the nucleotide metabolism (e.g. thymidine kinase) whose requirement is higher in tumor cells.
Herpes Simplex Virus 1 is a stranded DNA virus characterized by a 152-Kb linear genome, an icosaedral capsid, and a lipid envelope of approximately 200 nm diameter. The infection of humans with HSV1 is very common through saliva and skin of infected individuals, and after a lytic spread in the epithelium they can establish life-long latency in the innervating neurones. Eventually, virus reactivated from such neurons can travel to skin to produce lesions (sores) that are less severe than the initial primary infection due to the presence of antiviral immunity. Except in immune-suppressed patients, the infection with HSV1 is asymptomatic or produces benign blisters that heal spontaneously or that can be effectively treated with nucleoside analogs (e.g. acylovir) that target the viral thymidine kinase (TK) enzyme. This enzyme is essential for the virus replication in non-dividing (normal) cells. The idea of using a herpes virus deleted in the TK gene as an oncolytic virus sparked a new era of virotherapy with viruses designed to depend on the molecular changes that characterize cancer cells [5]. TK is the target of the anti-herpetic drugs, but the mutation of other virus genes needed to stimulate the synthesis of nucleotides in normal quiescent cells (UL39 or ICP6 gene encoding rubonucleotide reductase) was considered safe enough to allow for clinical development. The deletion of anti-IFN genes (ICP34.5) is another strategy to achieve tumor-selective HSV replication. ICP34.5 mutant HSV1716 and the double ICP34.5/ICP6 mutant G207 have been used in phase 1 clinical trials, but the double deletion substantially reduces virus replication in most tumor cells and the clinical activity of G207 has been much lower. However, it has been the combination of the high replication potency of the single ICP34.5 mutants with immunostimulatory strategies that has allowed clinical progress. Oncovex-GMCSF is a HSV1 where ICP34.5 has been replaced with the GM-CSFgene and it also contains a mutation in the ICP47 gene encoding a protein that binds to the transporter associated with antigen processing (TAP). In a phase 2 clinical study in 50 patients with advanced melanoma, repeated intratumor injections of Oncovex-GMCSF caused complete regressions of injected and uninjected tumors in eight patients [13]. Now this oncolytic HSV is in phase 3 clinical trials for metastatic melanoma and, together with reovirus Reolysin and Vaccinia virus JX-594, leads the expectations in virotherapy.
Vaccinia Virus is a DNA virus of the poxvirus family, characterized by a big lipid envelope (350 nm of diameter) containing a linear double-stranded DNA of 190 Kb. This complex virus contains its own RNA polymerase that allows a fast replication in the cytoplasm. Vaccinia virus was used widely until the early 70s to vaccinate against smallpox, which is produced by a similar poxvirus (the Variola Virus). Different strains of this virus, obtained by repeated passages in different cells in vivo or in vitro, were used before as vaccines and now to construct oncolytic viruses: Lister (in UK, Africa, Asia and Oceania), Dryvax, or Wyeth (USA). These strains are considered safe except in individuals suffering from immunosuppression, cardiac disease, or atopic dermatitis. As vaccination is no longer done since the early 70s, patients younger than 40 are expected to be naïve to the virus and superior oncolytic activity or toxicity may result. Jennerex-594 (JX-594) derives from the Wyeth strain modified with a TK gene deletion to allow selective replication in tumor cells, which increases safety. As Oncovex-GMCSF, JX-594 also expresses human GM-CSF for immunotherapy. JX-594 has demonstrated efficacy in intratumoral phase 2 trials in melanoma and hepatocellular carcinoma. However, it has focused major interest in the virotherapy field after a phase 1 trial of intravenous administration where it demonstrated cancer-selective and dose-related tumor targeting and antitumor effects [14]. Phase 2 trials of intravenous JX-594 for hepatocellular carcinoma, as well as trials with other oncolytic vaccinia viruses (vvDD-CDSR and GL-ONC1) are ongoing.
Adenovirus is a DNA virus characterized by a linear double-stranded genome of 36 Kb encapsidated in an icosahedral capsid of 100 nm diameter without lipid envelope. Among 52 human serotypes, type 5 is the most frequently used in virology studies and for oncolytic development. This virus often infects humans, with 50 % seropositivity worldwide, causing respiratory infections and conjunctivitis in children that can be severe when immunity is compromised, such after hematopoietic stem cell transplantation. In immunosuppressed patients adenovirus 5 infections causes diarrhea, trombocytopenia, transaminatis and bone marrow failure, and can be fatal at more than 10E10 virus copies per ml of blood, suggesting the adverse events and toxicity than can be expected from oncolytic adenoviruses. Adenovirus was used in virotherapy soon after being discovered [15]. In 1996, Frank Mc Kormick and colleagues proposed the use of adenovirus mutants unable to inactivate p53 (E1b-55K deletion mutant Onyx-015) to target p53-defective tumor cells [16]. The use of mutants defective in pRB-binding was later proposed to restrict replication to tumor cells defective in the pRB pathway [17, 18]. From the safety standpoint, adenovirus is unique as it presents two traits that allow the design of tumor-selectivity: i) replication in quiescent cells is highly dependent on the inactivation of tumor-suppressor proteins by virus early proteins, and the deletion of these functions lead to a selectivity based on tumor-suppressor gene defects; and ii) the transcriptional control of all virus genes is regulated by E1a and replacing the E1a promoter with tumor-selective promoters results in highly selective oncolytic viruses. However, clinical trials with intravenous or intratumoral oncolytic adenoviruses have not been successful [19, 20]. As with Herpes and Vaccinia Virus, arming the virus with genes for enhanced potency or immunostimulation may be a requirement.
Considerations for clinical trials
The current third wave of virotherapy has reached the clinical test very notoriously. Early in 2012 there were 34 clinical trials recruiting patients for oncolytic virus injections, most of them intravenously [21]. Reovirus, Vaccinia Virus, Measles virus, Herpes Simplex virus, and Adenovirus are leading the ranking of frequency, followed by isolated trials recruiting for Coxsackie virus, Seneca Valley virus, Retrovirus, Vesicular Stomatitis virus, Poliovirus, and Parvovirus. One Reovirus (Reolysin, Oncolytic Biotech) and one Herpes virus (Talimogene laherparepvec or Oncovex-GMCSF, Amgen) are the two agents most advanced in clinical development in phase 3 trials. The combination with chemotherapy is most frequent. Several considerations are worth discussing for clinical trials. Compared with other drugs, oncolytic viruses show an inverse pharmacokinetics. Viruses can burst from tumors to give secondary peaks of viremia that indicate virus replication. These bursts of progeny can result in delayed activity and toxicity that are not dose-dependent. The toxicity may correlate better with the amount of tumor cells (tumor load of the patient) than with the initially injected dose. Therefore, it would be safer to proceed from patients with a low tumor burden to patients with large metastatic disease to detect possible adverse events. In addition, the fast virus-induced tumor lysis can result in a tumor lysis syndrome and a cytokine storm, which are expected to correlate with the patient tumor load. With regard to efficacy, anti-virus immune responses may prevent the efficacy of repeated dosing, in particular upon systemic administration. Despite this, almost all protocols are based on repeated administrations following the schemes of radio and chemotherapy. Immunotherapy experience with dendritic cells or tumor antigens vaccines shows that responses may develop eventually after multiple ineffective rounds, indicating that sparking the proper immune response may depend on a rare encounter of the antigen-presenting cells and the presumably few tumor-specific CD4 and CD8 lymphocytes. So if elicitation of an anti-tumoral immune response is sought by arming the oncolytic virus with stimulatory genes or tumor antigens, repeating injections makes sense even if oncolysis is expected to be quickly neutralized by previous virus administrations. Such perseverance can yield responses (complete and partial) even after 2 years of treatment [13]. As performed in most prime-boost immunotherapies, alternating different oncolytic virus would be ideal to stimulate immunity against the tumor in spite of the immunity against the virus. The immune response may also preclude the interpretation of conventional antitumor response criteria (RECIST). For example, swelling of metastases is a typical symptom a successful immunotherapy and the size increase or the unveiling as metastases can be taken as tumor progression (defined as pseudo-progression) [13, 22]. Another consideration for efficacy is the likely synergy with chemotherapy and radiotherapy [23]. Viruses often increase sensitivity to chemo and radiotherapy and chemo and radiotherapy increase virus replication. This combination may thus increase clinical benefit.
Major hurdles for clinical efficacy
Despite the hopes on the new wave of virotherapy, major hurdles need to be overcome to achieve efficacy. Reaching the maximum number of tumor cells via systemic administration will be a great advantage against metastatic cancer. Even for those who rely on immunotherapy, the local reversion of tumor immunosuppression by the oncolytic virus requires tumor targeting. The neutralizing interaction with antibodies and other blood proteins and cells, and the loss of virus through the vascular fenestrations of spleen and liver (150 nm diameter) with the subsequent uptake by macrophages (Kupffer cells in liver) are key to improve virus bioavailability for tumor cells. This systemic availability is higher for Herpesvirus and Vaccinia Virus as their envelope derive from infected cells and it has a diameter larger than the fenestrations. Large particles compatible with blood components show a natural tropism for tumors due to the enhanced permeability and retention (EPR) associated with the disorganized tumor vasculature [24]. For adenovirus, the mutation of the capsid binding sites to blood factors and cells and to scavenger receptors on macrophages may improve bioavailability and passive tumor targeting [25]. However, these interactions are just recently being characterized and a more radical solution may be the transport of the infectious virus genome in non-viral carriers such as liposomes, nanoparticles, or carrier cells [26]. Non viral delivery with vectors that do not elicit immune responses may also improve the prospects of repeated dosing. A second key limitation is the intratumoral spread or dissemination of the virus. Tumors are characterized by a stroma composed of extracellular matrix proteins (collagen, fibronectin, laminin, fibrin, and sparc/osteonectin), polysaccharides (proteoglycan glycosaminoglycans such as protein-attached heparan, condroitin and keratan sulfates, and non-proteoglycan glycosaminoglycans such as hyaluronan), and cells such as fibroblasts and inflammatory cells. In contrast to the tumor targeting problem, for intratumoral dissemination the smaller the virus the better, with the small Picornaviruses and Coxsackie Viruses having a much better diffusion coefficients than Vaccinia and Herpes Viruses. The stroma not only precludes virus spread but also virus arrival to the tumor via the vasculature given that the stroma, together with the poor lymphatic drainage in tumors, increases the interstitial pressure and generates a lymph flow against virus extravasation and diffusion towards the tumor. Therefore, arming the viruses with stroma-degrading enzymes and extending virus permissiveness to non-tumor stromal cells is currently actively explored to improve virus spread and virus repeated delivery. However, clinical experience in the past has indicated that the major barrier to virotherapy is the anti-virus immune response. Although the tumor environment is immunosuppressive, the danger signals elicited by virus replication are so strong that virus is detected and neutralized. There is hope that the lysis of tumor cells and release of tumor antigens within the new immuno-stimulatory environment created by the oncolytic virus may allow for a response against the tumor [27]. Indeed the most clinically advanced oncolytic viruses are armed with GM-CSF. However, clinical experience shows that while virus rejection is usual, tumor rejection is very rare. This may be related to the immunodominance of virus antigens against tumor antigens [28]. Compared with virus proteins, tumor antigens are normal proteins with minor changes, overexpressed, or expressed out of their normal site or time and thus, a higher immunotolerance to these proteins is expected. As the cellular adaptive immune response focuses on a few epitopes at a time, the response against the most immunogenic or dominant epitopes precludes the response against the rest of epitopes. General immunostimulatory protocols or genes will not change the immunodominance of the virus. Priming immunity against the tumor antigen or tolerizing against the virus antigens may, on the contrary, help to overcome the virus dominance.
Taking these delivery, spread, and immunity hurdles into account may lead to the success of virotherapy. In the past 20 years, we have seen a new wave of viruses designed to replicate selectively in tumor cells and to stimulate the immune system. In the next 10 years, we will see capsid-modified viruses armed with matrix-degrading and immunostimulatory genes. In the next 20 years, it is conceivable to obtain nonviral vectors that deliver infectious virus genomes encoding stroma-degrading genes and genes that selectively enhance the immune response against the tumor but not against the virus.
References
Javier RT, Butel JS (2008) The history of tumor virology. Cancer Res 68:7693–7706
Sinkovics J, Horvath J, Szabo-Szabari M (1994) Human cancer vaccines. Leukemia 8(Suppl 1):S194–S197
Kelly E, Russell SJ (2007) History of oncolytic viruses: genesis to genetic engineering. Mol Ther 15:651–659
De Pace N (1912) Sulla scomparsa di un enorme cranco vegetante del collo dell′utero senza cura chirurgica. Ginecologia 9:82–89
Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM (1991) Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 252:854–856
Alemany R, Cascallo M (2009) Oncolytic adenoviruses from the perspective of the immune system. Future Microbiol 4:527–536
Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C, Newbold K, Gore ME, Larkin J, Syrigos KN, Coffey M, Thompson B, Mettinger K, Vile RG, Pandha HS, Hall GD, Melcher AA, Chester J, Harrington KJ (2012) Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res 18:2080–2089
Yaacov B, Lazar I, Tayeb S, Frank S, Izhar U, Lotem M, Perlman R, Ben-Yehuda D, Zakay-Rones Z, Panet A (2012) Extracellular matrix constituents interfere with Newcastle disease virus spread in solid tissue and diminish its potential oncolytic activity. J Gen Virol 93:1664–1672
Hotte SJ, Lorence RM, Hirte HW, Polawski SR, Bamat MK, O’Neil JD, Roberts MS, Groene WS, Major PP (2007) An optimized clinical regimen for the oncolytic virus PV701. Clin Cancer Res 13:977–985
Lam HY, Yeap SK, Rasoli M, Omar AR, Yusoff K, Suraini AA, Alitheen NB (2011) Safety and clinical usage of newcastle disease virus in cancer therapy. J Biomed Biotechnol 2011:718710
Galanis E (2010) Therapeutic potential of oncolytic measles virus: promises and challenges. Clin Pharmacol Ther 88:620–625
Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, Kaur JS, Haluska PJ Jr, Aderca I, Zollman PJ, Sloan JA, Keeney G, Atherton PJ, Podratz KC, Dowdy SC, Stanhope CR, Wilson TO, Federspiel MJ, Peng KW, Russell SJ (2010) Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res 70:875–882
Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, Gonzalez R, Glaspy J, Whitman E, Harrington K, Goldsweig H, Marshall T, Love C, Coffin R, Nemunaitis JJ (2009) Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 27:5763–5771
Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R, Pelusio A, Le Boeuf F, Burns J, Evgin L, De Silva N, Cvancic S, Robertson T, Je JE, Lee YS, Parato K, Diallo JS, Fenster A, Daneshmand M, Bell JC, Kirn DH (2011) Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477:99–102
Smith RR, Huebner RJ, Rowe WP, Schatten WF, Thomas LB (1956) Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer 9:1211–1218
Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F (1996) An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373–376
Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP (2000) A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 19:2–12
Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawkins L, Kirn D (2000) An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med 6:1134–1139
Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, Yu DC, Aimi J, Ando D, Working P, Kirn D, Wilding G (2006) A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther 14:107–117
Pesonen S, Kangasniemi L, Hemminki A (2011) Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm 8:12–28
Russell SJ, Peng KW, Bell JC (2012) Oncolytic virotherapy. Nat Biotechnol 30:658–670
Ribas A, Chmielowski B, Glaspy JA (2009) Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res 15:7116–7118
Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA (2010) Intelligent design: combination therapy with oncolytic viruses. Mol Ther 18:251–263
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–151
Coughlan L, Alba R, Parker AL, Bradshaw AC, McNeish IA, Nicklin SA, Baker AH (2011) Tropism-modification strategies for targeted gene delivery using adenoviral vectors. Viruses 2:2290–2355
Choi JW, Lee JS, Kim SW, Yun CO (2012) Evolution of oncolytic adenovirus for cancer treatment. Adv Drug Deliv Rev 64:720–729
Melcher A, Parato K, Rooney CM, Bell JC (2011) Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther 19:1008–1016
Akram A, Inman RD (2012) Immunodominance: a pivotal principle in host response to viral infections. Clin Immunol 143:99–115
Acknowledgments
Thanks to Cristina Balagué for critical reading. Dedicated to the memory of Dr. Alexander Pereboev.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alemany, R. Viruses in cancer treatment. Clin Transl Oncol 15, 182–188 (2013). https://doi.org/10.1007/s12094-012-0951-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0951-7