Abstract
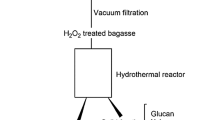
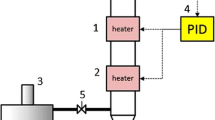
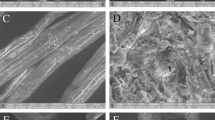
The complex chemical and structural arrangement of lignocellulosic biomass demand a pretreatment step to dismantle its coherent nature. The current study was designed to study H2SO4 impregnation before hydrothermal pretreatment (HP) of sugarcane bagasse for biogas production using response surface methodology based on defining factors: temperature (160, 180, 200 °C), time (5, 12, 19 min), and H2SO4 concentration (1, 2, 3% w/v). The H2SO4 impregnation had a paramount impact in combination with temperature and treatment time parameters. The lowest solid yield 12.3% was recorded in pretreatment run A-HSO (200 °C, 19 min, 3% H2SO4), up to 100% xylan removal was noted in four pretreatment runs (A-HSO; 200 °C, 19 min, 3% H2SO4, B-HSO; 200 °C, 19 min, 1% H2SO4, C-HSO; 200 °C, 5 min, 3% H2SO4, and O-HSO; 213.6 °C, 12 min, 2% H2SO4), maximum klason lignin increase in insoluble solid fraction (ISF) verified was 358.85% in pretreatment run O-HSO at 213.6 °C, while glucan content reduction (33.18–74.14%) was also observed in pretreatment runs A-HSO, C-HSO, and O-HSO. Results verified maximum methane recovery of 160.16 NmL (g TVS)−1 in pretreatment run I-SHO (180 °C, 12 min, 2% H2SO4). Microbial community analysis observed the predominance of Flavobacterium and Desulfosporosinus genera of Bacterial domain and Methanolinea of Archaea domain. H2SO4 impregnation before HP though not suitable for methane production from ISF could be employed in a biorefinery perspective to obtain valuables (chemicals and fuels) due to the production of organic acids rich liquid stream and lignin-rich solid fraction.
Graphical Abstract







Similar content being viewed by others
Abbreviations
- APHA:
-
American Public Health Association
- BMP:
-
Biomethane potential
- CCD:
-
Central composite design
- COD:
-
Chemical oxygen demand
- DM:
-
Dry matter
- GAIN:
-
Global Agricultural Information Network
- HP:
-
Hydrothermal pretreatment
- HPLC:
-
High-performance liquid chromatography
- NCBI:
-
National Center for Biotechnology Information
- OTU:
-
Operational taxonomic unit
- PCR:
-
Polymerase chain reaction
- RDP:
-
Ribosomal Database Project
- SB:
-
Sugarcane bagasse
- SEM:
-
Scanning electron microscopy
- TCD:
-
Thermal conductivity detector
- TS:
-
Total solids
- TVS:
-
Total volatile solids
References
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Dell RM, Rand DAJ (2001) Energy storage — a key technology for global energy sustainability. J Power Sources 100:2–17. https://doi.org/10.1016/S0378-7753(01)00894-1
Yaman S (2004) Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers Manag 45:651–671. https://doi.org/10.1016/S0196-8904(03)00177-8
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861. https://doi.org/10.1016/j.biortech.2009.11.093
Carrere H, Antonopoulou G, Affes R, Passos F, Battimelli A, Lyberatos G, Ferrer I (2016) Review of feedstock pretreatment strategies for improved anaerobic digestion: from lab-scale research to full-scale application. Bioresour Technol 199:386–397. https://doi.org/10.1016/j.biortech.2015.09.007
Bušić A, Kundas S, Morzak G et al (2018) Recent trends in biodiesel and biogas production. Food Technol Biotechnol 56:152–173. https://doi.org/10.17113/ftb.56.02.18.5547
Barros S (2018) Brazil - Sugar Annual. Global Agricultural Information Network Report, USDA Foreign Agricultural Service https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Sugar%20Annual_Sao%20Paulo%20ATO_Brazil_4-13-2018.pdf. Accessed 24 March 2021
Canilha L, Chandel AK, Suzane dos Santos Milessi T et al (2012) Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J Biomed Biotechnol 2012:15–15. https://doi.org/10.1155/2012/989572
Ruiz HA, Conrad M, Sun S-N, Sanchez A, Rocha GJM, Romaní A, Castro E, Torres A, Rodríguez-Jasso RM, Andrade LP, Smirnova I, Sun RC, Meyer AS (2020) Engineering aspects of hydrothermal pretreatment: from batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour Technol 299:122685. https://doi.org/10.1016/j.biortech.2019.122685
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36:2328–2342. https://doi.org/10.1016/j.energy.2011.03.013
Ahmad F, Silva EL, Varesche MBA (2018) Hydrothermal processing of biomass for anaerobic digestion – a review. Renew Sust Energ Rev 98:108–124. https://doi.org/10.1016/j.rser.2018.09.008
Torres AI, Ashraf MT, Chaturvedi T, Schmidt JE, Stephanopoulos G (2017) Hydrothermal pretreatment: process modeling and economic assessment within the framework of biorefinery processes. In: Ruiz HA, Hedegaard Thomsen M, Trajano HL (eds) Hydrothermal Processing in Biorefineries: Production of Bioethanol and High Added-Value Compounds of Second and Third Generation Biomass. Springer International Publishing, Cham, pp 207–235
Suriyachai N, Weerasai K, Upajak S, Khongchamnan P, Wanmolee W, Laosiripojana N, Champreda V, Suwannahong K, Imman S (2020) Efficiency of catalytic liquid hot water pretreatment for conversion of corn stover to bioethanol. ACS Omega 5:29872–29881. https://doi.org/10.1021/acsomega.0c04054
Dimitrellos G, Lyberatos G, Antonopoulou G (2020) Does acid addition improve liquid hot water pretreatment of lignocellulosic biomass towards biohydrogen and biogas production? Sustainability 12:8935. https://doi.org/10.3390/su12218935
Wang D, Shen F, Yang G, Zhang Y, Deng S, Zhang J, Zeng Y, Luo T, Mei Z (2018) Can hydrothermal pretreatment improve anaerobic digestion for biogas from lignocellulosic biomass? Bioresour Technol 249:117–124. https://doi.org/10.1016/j.biortech.2017.09.197
Hashemi SS, Karimi K, Mirmohamadsadeghi S (2019) Hydrothermal pretreatment of safflower straw to enhance biogas production. Energy 172:545–554. https://doi.org/10.1016/j.energy.2019.01.149
Gong L, Yang X, Wang Z, Zhou J, You X (2019) Impact of hydrothermal pre-treatment on the anaerobic digestion of different solid–liquid ratio sludges and kinetic analysis. RSC Adv 9:19104–19113. https://doi.org/10.1039/C9RA01662G
Ahmad F, Sakamoto IK, Adorno MAT, Motteran F, Silva EL, Varesche MBA (2018) Methane production from hydrogen peroxide assisted hydrothermal pretreatment of solid fraction sugarcane bagasse. Waste Biomass Valorization 11:31–50. https://doi.org/10.1007/s12649-018-0452-1
Marasabessy A, Kootstra AMJ, Sanders JP, Weusthuis RA (2012) Dilute H2SO4-catalyzed hydrothermal pretreatment to enhance enzymatic digestibility of Jatropha curcas fruit hull for ethanol fermentation. Int J Energy Environ Eng 3:15. https://doi.org/10.1186/2251-6832-3-15
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2006) Determination of sugars, byproducts, and degradation products in liquid fraction process samples. NREL. https://www.nrel.gov/docs/gen/fy08/42623.pdf. Accessed 24 March 2021
Motteran F, Braga JK, Sakamoto IK, Varesche MBA (2014) Methanogenic potential of an anaerobic sludge in the presence of anionic and nonionic surfactants. Int Biodeterior Biodegrad 96:198–204. https://doi.org/10.1016/j.ibiod.2014.10.001
Tsuchida JE, Rezende CA, de Oliveira-Silva R, Lima MA, d’Eurydice MN, Polikarpov I, Bonagamba TJ (2014) Nuclear magnetic resonance investigation of water accessibility in cellulose of pretreated sugarcane bagasse. Biotechnol Biofuels 7:7. https://doi.org/10.1186/s13068-014-0127-5
Caza N, Bewtra JK, Biswas N, Taylor KE (1999) Removal of phenolic compounds from synthetic wastewater using soybean peroxidase. Water Res 33:3012–3018. https://doi.org/10.1016/S0043-1354(98)00525-9
Zinder SH, Cardwell SC, Anguish T, Lee M, Koch M (1984) Methanogenesis in a thermophilic (58 °C) anaerobic digestor: Methanothrix sp. as an important aceticlastic methanogen. Appl Environ Microbiol 47:796–807
Hansen TL, Schmidt JE, Angelidaki I, Marca E, Jansen JC, Mosbæk H, Christensen TH (2004) Method for determination of methane potentials of solid organic waste. Waste Manag 24:393–400. https://doi.org/10.1016/j.wasman.2003.09.009
Zwietering MH, Jongenburger I, Rombouts FM, van ’t Riet K (1990) Modeling of the bacterial growth curve. Appl Environ Microbiol 56:1875–1881
Larnaudie V, Ferrari MD, Lareo C (2019) Enzymatic hydrolysis of liquid hot water-pretreated switchgrass at high solid content. Energy Fuel 33:4361–4368. https://doi.org/10.1021/acs.energyfuels.9b00513
Zhang Z, Rackemann DW, Doherty WOS, O’Hara IM (2013) Glycerol carbonate as green solvent for pretreatment of sugarcane bagasse. Biotechnol Biofuels 6:153. https://doi.org/10.1186/1754-6834-6-153
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66:5488–5491. https://doi.org/10.1128/AEM.66.12.5488-5491.2000
Walters WA, Caporaso JG, Lauber CL et al (2011) PrimerProspector: de novo design and taxonomic analysis of barcoded polymerase chain reaction primers. Bioinformatics (Oxford, England) 27:1159–1161. https://doi.org/10.1093/bioinformatics/btr087
Lu XB, Zhang YM, Angelidaki I (2009) Optimization of H2SO4-catalyzed hydrothermal pretreatment of rapeseed straw for bioconversion to ethanol: focusing on pretreatment at high solids content. Bioresour Technol 100:3048–3053. https://doi.org/10.1016/j.biortech.2009.01.008
Yang HY, Wang K, Xu F, Sun RC, Lu Y (2012) H2SO4-catalyzed hydrothermal pretreatment of triploid poplar to enhance enzymatic hydrolysis. Ind Eng Chem Res 51:11598–11604. https://doi.org/10.1021/ie300895y
Sakaki T, Shibata M, Sumi T, Yasuda S (2002) Saccharification of cellulose using a hot- compressed water-flow reactor. Ind Eng Chem Res 41:661–665. https://doi.org/10.1021/ie010614s
Rocha GJM, Silva VFN, Martin C et al (2013) Effect of xylan and lignin removal by hydrothermal pretreatment on enzymatic conversion of sugarcane bagasse cellulose for second generation ethanol production. Sugar Tech 15:390–398
Senila L, Senila M, Costiug S, Miclean M, Cerasel V, Roman C (2014) The autohydrolysis of Albies Alba wood using adaptive neural fuzzy interference system mathematical modeling. Int J Green Energy 11:611–624. https://doi.org/10.1080/15435075.2013.777907
Xiao L-P, Sun Z-J, Shi Z-J et al (2011) Impact of hot compressed water pretreatment on the structural changes of woody biomass for bioethanol prodution. Biorsources 6:1576–1598
Hu F, Ragauskas A (2012) Pretreatment and lignocellulosic chemistry. Bioenerg Res 5:1043–1066. https://doi.org/10.1007/s12155-012-9208-0
Ramos LP (2003) The chemistry involved in the steam treatment of lignocellulosic materials. Quim Nova 26:863–871. https://doi.org/10.1590/S0100-40422003000600015
Nitsos CK, Choli-Papadopoulou T, Matis KA, Triantafyllidis KS (2016) Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustain Chem Eng 4:4529–4544. https://doi.org/10.1021/acssuschemeng.6b00535
Kumar R, Hu F, Sannigrahi P, Jung S, Ragauskas AJ, Wyman CE (2013) Carbohydrate derived-pseudo-lignin can retard cellulose biological conversion. Biotechnol Bioeng 110:737–753. https://doi.org/10.1002/bit.24744
Kim KH, Tucker M, Nguyen Q (2005) Conversion of bark-rich biomass mixture into fermentable sugar by two-stage dilute acid-catalyzed hydrolysis. Bioresour Technol 96:1249–1255. https://doi.org/10.1016/j.biortech.2004.10.017
Li H, Pu Y, Kumar R, Ragauskas AJ, Wyman CE (2014) Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms. Biotechnol Bioeng 111:485–492. https://doi.org/10.1002/bit.25108
Donohoe BS, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2008) Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol Bioeng 101:913–925. https://doi.org/10.1002/bit.21959
Ahring BK, Biswas R, Ahamed A, Teller PJ, Uellendahl H (2015) Making lignin accessible for anaerobic digestion by wet-explosion pretreatment. Bioresour Technol 175:182188–182188. https://doi.org/10.1016/j.biortech.2014.10.082
Kobayashi F, Take H, Asada C, Nakamura Y (2004) Methane production from steam-exploded bamboo. J Biosci Bioeng 97:426–428. https://doi.org/10.1016/S1389-1723(04)70231-5
Liew LN, Shi J, Li Y (2012) Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 46:125–132. https://doi.org/10.1016/j.biombioe.2012.09.014
Monlau F, Sambusiti C, Barakat A, Quéméneur M, Trably E, Steyer JP, Carrère H (2014) Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol Adv 32:934–951. https://doi.org/10.1016/j.biotechadv.2014.04.007
Ribeiro FR, Passos F, Gurgel LVA, Baêta BEL, de Aquino SF (2017) Anaerobic digestion of hemicellulose hydrolysate produced after hydrothermal pretreatment of sugarcane bagasse in UASB reactor. Sci Total Environ 584–585:1108–1113. https://doi.org/10.1016/j.scitotenv.2017.01.170
Haandel A, Lettinga G (1994) Anaerobic sewage treatment: a practical guide for regions with a hot climate. John Wiley & Sons, Chichester UK
Niladevi KN, Sukumaran RK, Jacob N, Anisha GS, Prema P (2009) Optimization of laccase production from a novel strain—streptomyces psammoticus using response surface methodology. Microbiol Res 164:105–113. https://doi.org/10.1016/j.micres.2006.10.006
Mizuno O, Li MYY, Noike MT (1998) The behavior of sulfate-reducing bacteria in acidogenic phase of anaerobic dgiestion. Water Res 32:1626–1634
Weijma J, Gubbels F, Hulshoff Pol LW, Stams AJM, Lens P, Lettinga G (2002) Competition for H2 between sulfate reducers, methanogens and homoacetogens in a gas-lift reactor. Water Sci Technol 45:75–80
Muyzer G, Stams AJM (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6:441–454
Fernandez-Cegri V, De la Rubia MA, Raposo F, Borja R (2012) Effect of hydrothermal pretreatment of sunflower oil cake on biomethane potential focusing on fibre composition. Bioresour Technol 123:424–429. https://doi.org/10.1016/j.biortech.2012.07.111
O-Thong S, Boe K, Angelidaki I (2012) Thermophilic anaerobic co-digestion of oil palm empty fruit bunches with palm oil mill effluent for efficient biogas production. Appl Energy 93:648–654. https://doi.org/10.1016/j.apenergy.2011.12.092
Chandra R, Takeuchi H, Hasegawa T (2012) Hydrothermal pretreatment of rice straw biomass: a potential and promising method for enhanced methane production. Appl Energy 94:129–140. https://doi.org/10.1016/j.apenergy.2012.01.027
Zhang Q, Tang L, Zhang J, Mao Z, Jiang L (2011) Optimization of thermal-dilute sulfuric acid pretreatment for enhancement of methane production from cassava residues. Bioresour Technol 102:3958–3965. https://doi.org/10.1016/j.biortech.2010.12.031
Barakat A, Monlau F, Steyer J-P, Carrere H (2012) Effect of lignin-derived and furan compounds found in lignocellulosic hydrolysates on biomethane production. Bioresour Technol 104:90–99. https://doi.org/10.1016/j.biortech.2011.10.060
Chen Q, Chen K, Wang K, Ma J, Yang H, Chen J (2018) The effects of time and temperature in hydrothermal pretreatment on the enzymatic efficiency of wheat straw. BioResources 13:5193-5203
Ksiazek M, Karim AY, Bryzek D, Enghild JJ, Thøgersen IB, Koziel J, Potempa J (2015) Mirolase, a novel subtilisin-like serine protease from the periodontopathogen Tannerella forsythia. Biol Chem 396:261–275. https://doi.org/10.1515/hsz-2014-0256
Lawrence KA, Harris TM, Salter SJ, Hall RW, Smith-Vaughan HC, Chang AB, Marsh RL (2019) Method for culturing Candidatus Ornithobacterium hominis. J Microbiol Methods 159:157–160. https://doi.org/10.1016/j.mimet.2019.03.006
Seruga P, Krzywonos M, Paluszak Z, Urbanowska A, Pawlak-Kruczek H, Niedźwiecki Ł, Pińkowska H (2020) Pathogen reduction potential in anaerobic digestion of organic fraction of municipal solid waste and food waste. Molecules 25. https://doi.org/10.3390/molecules25020275
Bernardet J, Bowman J (2015) Flavobacterium. Bergey's Manual of Systematics of Archaea and Bacteria 1-75. https://doi.org/10.1002/9781118960608.gbm00312
Koga S, Ogawa J, Choi Y, Shimizu S (1999) Novel bacterial peroxidase without catalase activity from Flavobacterium meningosepticum: purification and characterization. Biochim Biophys Acta 1435:117–126
Kato S, Chino K, Kamimura N, Masai E, Yumoto I, Kamagata Y (2015) Methanogenic degradation of lignin-derived monoaromatic compounds by microbial enrichments from rice paddy field soil. Sci Rep. https://doi.org/10.1038/srep14295
Fowler SJ, Gutierrez-Zamora M-LL, Manefield M, Gieg LM (2014) Identification of toluene degraders in a methanogenic enrichment culture. FEMS Microbiol Ecol 89:625–636. https://doi.org/10.1111/1574-6941.12364
Kuppardt A, Kleinsteuber S, Vogt C, Lüders T, Harms H, Chatzinotas A (2014) Phylogenetic and functional diversity within toluene-degrading, sulphate-reducing consortia enriched from a contaminated aquifer. Microb Ecol 68:222–234. https://doi.org/10.1007/s00248-014-0403-8
Pobeheim H, Munk B, Müller H, Berg G, Guebitz GM (2010) Characterization of an anaerobic population digesting a model substrate for maize in the presence of trace metals. Chemosphere 80:829–836. https://doi.org/10.1016/j.chemosphere.2010.06.011
Anderson K, Sallis P, Uyanik S (2003) 24 - Anaerobic treatment processes. In: Handbook of Water and Wastewater Microbiology. Academic Press, London, pp 391–426
Delforno TP, Lacerda Júnior GV, Noronha MF et al (2017) Microbial diversity of a full-scale UASB reactor applied to poultry slaughterhouse wastewater treatment: integration of 16S rRNA gene amplicon and shotgun metagenomic sequencing. Microbiologyopen 6. https://doi.org/10.1002/mbo3.443
Del Nery V, Pozzi E, Damianovic M, Domingues M, Zaiat M (2008) Granules characteristics in the vertical profile of a full-scale upflow anaerobic sludge blanket reactor treating poultry slaughterhouse wastewater. Bioresour Technol 99:2018-2024. https://doi.org/10.1016/j.biortech.2007.03.019
Senés-Guerrero C, Colón-Contreras FA, Reynoso-Lobo JF, Tinoco-Pérez B, Siller-Cepeda JH, Pacheco A (2019) Biogas-producing microbial composition of an anaerobic digester and associated bovine residues. Microbiologyopen 8. https://doi.org/10.1002/mbo3.854
Bonnin AS, Boone DR (2006) The order Methanobacteriales. In: Dworkin M, Falkow S, Rosenberg E, et al (eds) The Prokaryotes: Archaea. Bacteria: Firmicutes, Actinomycetes. Springer, Singapore
Tabatabaei M, Rahim RA, Abdullah N, Wright ADG, Shirai Y, Sakai K, Sulaiman A, Hassan MA (2010) Importance of the methanogenic archaea populations in anaerobic wastewater treatments. Process Biochem 45:1214–1225. https://doi.org/10.1016/j.procbio.2010.05.017
Boone D (2015) Methanobacterium. Bergey's Manual of Systematics of Archaea and Bacteria 1-8. https://doi.org/10.1002/9781118960608.gbm00495
Vítězová M, Kohoutová A, Vítěz T, Hanišáková N, Kushkevych I (2020) Methanogenic microorganisms in industrial wastewater anaerobic treatment. Processes 8:1546. https://doi.org/10.3390/pr8121546
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministry of Education, Brazil, under the grant ID 23699920852.
Author information
Authors and Affiliations
Contributions
FA, ELS, and MBAV conceived and designed the study. FA conducted the experiments and wrote the manuscript. VS helped in conducting BMP experiments. IKS helped in molecular biology experiments. MBAV, IKS, and JS proofread the manuscript. All authors provided feedback on the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable
Consent for Publication
Not Applicable
Availability of Data and Material
All data is available on request.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, F., Silva, V., Sakamoto, I.K. et al. Bioprospecting Sulfuric Acid Assisted Hydrothermal Pretreatment of Sugarcane Bagasse and Microbial Community Structure for Methane Production. Bioenerg. Res. 15, 631–649 (2022). https://doi.org/10.1007/s12155-021-10268-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10268-2