Abstract
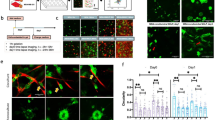
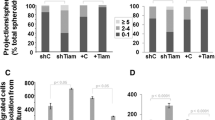
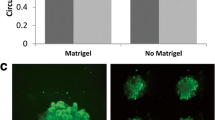
As cancer progresses, cells must adapt to a new and stiffer environment, which can ultimately alter how normal cells within the tumor behave. In turn, these cells are known to further aid tumor progression. Therefore, there is potentially a unique avenue to better understand metastatic potential through single-cell biophysical assays performed on patient-derived cells. Here, we perform biophysical characterization of primary human fibroblastic cells obtained from mammary carcinoma and normal contralateral tissue. Through a series of tissue dissociation, differential centrifugation and trypsinization steps, we isolate an adherent fibroblastic population viable for biomechanical testing. 2D TFM and 3D migration measurements in a collagen matrix show that fibroblasts obtained from patient tumors generate more traction forces and display improved migration potential than their counterparts from normal tissue. Moreover, through the use of an embedded spheroid model, we confirmed the extracellular matrix remodeling behavior of primary cells isolated from carcinoma. Overall, correlating biophysical characterization of normal- and carcinoma-derived samples from individual patients along with patient outcome may become a powerful approach to further our comprehension of metastasis and ultimately design drug targets on a patient-specific basis.




Similar content being viewed by others
References
Agus, D. B., J. F. Alexander, W. Arap, et al. A physical sciences network characterization of non-tumorigenic and metastatic cells. Sci. Rep. 3:1449, 2013.
Bordeleau, F., T. A. Alcoser, and C. A. Reinhart-King. Physical biology in cancer. 5. The rocky road of metastasis: the role of cytoskeletal mechanics in cell migratory response to 3D matrix topography. Am. J. Physiol. Cell Physiol. 306(2):C110–C120, 2014.
Bourhis, X. D.-L., Y. Berthois, G. Millot, et al. Effect of stromal and epithelial cells derived from normal and tumorous breast tissue on the proliferation of human breast cancer cell lines in co-culture. Int. J. Cancer 71(1):42–48, 1997.
Bremnes, R. M., T. Donnem, S. Al-Saad, et al. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 6(1):209–217, 2011.
Butcher, D. T., T. Alliston, and V. M. Weaver. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9(2):108–122, 2009.
Carey, S. P., T. M. D’Alfonso, S. J. Shin, and C. A. Reinhart-King. Mechanobiology of tumor invasion: engineering meets oncology. Crit. Rev. Oncol. Hematol. 83(2):170–183, 2012.
Carey, S. P., C. M. Kraning-Rush, R. M. Williams, and C. A. Reinhart-King. Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials 33(16):4157–4165, 2012.
Carey, S. P., A. Starchenko, A. L. McGregor, and C. A. Reinhart-King. Leading malignant cells initiate collective epithelial cell invasion in a three-dimensional heterotypic tumor spheroid model. Clin. Exp. Metastasis 30(5):615–630, 2013.
Cukierman, E., and D. E. Bassi. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin. Cancer Biol. 20(3):139–145, 2010.
De Wever, O., P. Demetter, M. Mareel, and M. Bracke. Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer 123(10):2229–2238, 2008.
Dembo, M., and Y. L. Wang. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 76(4):2307–2316, 1999.
Discher, D. E., P. Janmey, and Y. L. Wang. Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751):1139–1143, 2005.
Dumont, N., B. Liu, R. A. Defilippis, et al. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia 15(3):249–262, 2013.
Eriksson, J. E., T. Dechat, B. Grin, et al. Introducing intermediate filaments: from discovery to disease. J. Clin. Invest. 119(7):1763–1771, 2009.
Gaggioli, C., S. Hooper, C. Hidalgo-Carcedo, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9(12):1392–1400, 2007.
Gilbert, S., A. Loranger, N. Daigle, and N. Marceau. Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J. Cell Biol. 154(4):763–773, 2001.
Goetz, J. G., S. Minguet, I. Navarro-Lerida, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146(1):148–163, 2011.
Kawashiri, S., A. Tanaka, N. Noguchi, et al. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck 31(10):1346–1353, 2009.
Kraning-Rush, C. M., J. P. Califano, and C. A. Reinhart-King. Cellular traction stresses increase with increasing metastatic potential. PLoS One 7(2):e32572, 2012.
Kraning-Rush, C. M., S. P. Carey, J. P. Califano, B. N. Smith, and C. A. Reinhart-King. The role of the cytoskeleton in cellular force generation in 2D and 3D environments. Phys. Biol. 8(1):015009, 2011.
Levental, K. R., H. Yu, L. Kass, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139(5):891–906, 2009.
Lopez, J. I., I. Kang, W. K. You, D. M. McDonald, and V. M. Weaver. In situ force mapping of mammary gland transformation. Integr. Biol. (Camb) 3(9):910–921, 2011.
Madar, S., I. Goldstein, and V. Rotter. ‘Cancer associated fibroblasts’—more than meets the eye. Trends Mol. Med. 19(8):447–453, 2013.
Mao, Y., E. T. Keller, D. H. Garfield, K. Shen, and J. Wang. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 32(1–2):303–315, 2013.
Marsden, C. G., M. J. Wright, R. Pochampally, and B. G. Rowan. Breast tumor-initiating cells isolated from patient core biopsies for study of hormone action. Methods Mol. Biol. 590:363–375, 2009.
Orimo, A., P. B. Gupta, D. C. Sgroi, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121(3):335–348, 2005.
Paszek, M. J., N. Zahir, K. R. Johnson, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254, 2005.
Plodinec, M., M. Loparic, C. A. Monnier, et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 7(11):757–765, 2012.
Provenzano, P. P., K. W. Eliceiri, J. M. Campbell, D. R. Inman, J. G. White, and P. J. Keely. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4(1):38, 2006.
Provenzano, P. P., D. R. Inman, K. W. Eliceiri, S. M. Trier, and P. J. Keely. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys. J. 95(11):5374–5384, 2008.
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2014; http://www.R-project.org/.
Reinhart-King, C. A., M. Dembo, and D. A. Hammer. The dynamics and mechanics of endothelial cell spreading. Biophys. J. 89(1):676–689, 2005.
Ronnov-Jessen, L., O. W. Petersen, and M. J. Bissell. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol. Rev. 76(1):69–125, 1996.
Schedin, P., and P. J. Keely. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 3(1):a003228, 2011.
Shieh, A. C., H. A. Rozansky, B. Hinz, and M. A. Swartz. Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res. 71(3):790–800, 2011.
Shvetsova, E. V., O. S. Rogovaya, S. B. Tkachenko, I. V. Kiselev, A. V. Vasil’ev, and V. V. Terskikh. Contractile capacity of fibroblasts from different sources in the model of living skin equivalent. Biol. Bull. Russ. Acad. Sci. 35(2):146–150, 2008.
Speirs, V., A. R. Green, D. S. Walton, et al. Short-term primary culture of epithelial cells derived from human breast tumours. Br. J. Cancer 78(11):1421–1429, 1998.
Wolf, K., M. Te Lindert, M. Krause, et al. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 201(7):1069–1084, 2013.
Wolf, K., Y. I. Wu, Y. Liu, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 9(8):893–904, 2007.
Wood, S. N. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall/CRC, 2006.
Yamaguchi, H., N. Yoshida, M. Takanashi, et al. Stromal fibroblasts mediate extracellular matrix remodeling and invasion of scirrhous gastric carcinoma cells. PLoS One 9(1):e85485, 2014.
Zhang, W., L. M. Matrisian, K. Holmbeck, C. C. Vick, and E. L. Rosenthal. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer 6:52, 2006.
Acknowledgments
This work was supported by the Cornell Center on the Microenvironment & Metastasis through Award Number U54CA143876 from the National Cancer Institute, a National Science Foundation – National Institute of Health Physical and Engineering Sciences in Oncology (PESO) award (Award Number 1233827) and an NSF CAREER award to C. A. R. This work was also supported by National Science Foundation Graduate Research Fellowships to S. P. C. and M. C. L.
Conflict of interest
T. A. A., F. B., S. P. C., M. C. L., D. R. K., S. S., S. V., S. J. S., and C. A. R declare that they have no conflicts of interest.
Ethical Standards
All experiments using samples from human subjects were carried out in accordance with Weill Cornell IRB guidelines. Human tissues were de-identified. No animals were used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Cheng Dong oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Alcoser, T.A., Bordeleau, F., Carey, S.P. et al. Probing the Biophysical Properties of Primary Breast Tumor-Derived Fibroblasts. Cel. Mol. Bioeng. 8, 76–85 (2015). https://doi.org/10.1007/s12195-014-0360-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-014-0360-9