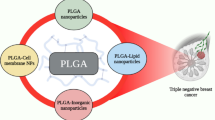
Abstract
Introduction
Triple negative breast cancer (TNBC) is a highly aggressive type of breast cancer with high resistance to current standard therapies. We demonstrate that phenotypically stratified carbon nanoparticle is highly effective in delivering a novel combinatorial triple drug formulation for synergistic regression of TNBC in vitro and in vivo.
Method
The combinatorial formulation is comprised of repurposed inhibitors of STAT3 (nifuroxazide), topoisomerase-II-activation-pathway (amonafide) and NFκb (pentoxifylline). Synergistic effect of drug combination was established in a panel of TNBC-lines comprising mesenchymal-stem-like, mesenchymal and basal-like cells along with non-TNBC-cells. The delivery of combinatorial drug formulation was achieved using a phenotypically screened carbon nanoparticles for TNBC cell lines.
Results
Results indicated a remarkable five-fold improvement (IC50-6.75 µM) from the parent drugs with a combinatorial index <1 in majority of the TNBC cells. Multi-compartmental carbon nanoparticles were then parametrically assessed based on size, charge (positive/negative/neutral) and chemistry (functionalities) to study their likelihood of crossing endocytic barriers from phenotypical standpoint in TNBC lines. Interestingly, a combination of clathrin mediated, energy and dynamin dependent pathways were predominant for sulfonated nanoparticles, whereas pristine and phospholipid particles followed all the investigated endocytic pathways.
Conclusions
An exactitude ‘omics’ approach helps to predict that phospholipid encapsulated-particles will predominantly accumulate in TNBC comprising the drug-‘cocktail’. We investigated the protein expression effects inducing synergistic effect and simultaneously suppressing drug resistance through distinct mechanisms of action.









Similar content being viewed by others
References
Allen, C., Y. Yu, A. Eisenberg, and D. Maysinger. Cellular internalization of PCL20-b-PEO44 block copolymer micelles. Biochim. Biophys. Acta. 1421:32, 1999.
Andersson, B. S., M. Beran, M. Bakic, L. E. Silberman, R. A. Newman, and L. A. Zwelling. In vitro toxicity and DNA cleaving capacity of benzisoquinonlinedione (nafimide; NSC 308847) in human leukemia. Cancer Res. 1987:47, 1040.
Arvizo, R. R., S. Rana, O. R. Miranda, R. Bhattacharya, V. M. Rotello, and P. Mukherjee. Mechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface charge. Nanomedicine. 7:580, 2011.
Brana, M. F., and A. M. Sanz. Synthesis and cytostatic activity of benz[de]isoqinolin-1,3-diones. Structure-activity relationships. Eur. J. Med. Chem. 16:207, 1981.
Brenton, J. D., L. A. Carey, A. A. Ahmed, and C. Caldas. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J. Clin. Oncol. 23:7350, 2005.
Brigger, I., C. Dubernet, and P. Couvreur. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 64:24, 2012.
Che-Ming, J. H., S. Aryal, and L. Zhang. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 1:323, 2010.
Chen, X., F. Tian, X. Zhang, and W. Wang. Internalization pathways of nanoparticles and their interaction with a vesicle. Soft Matter. 9:7592, 2013.
Chen, Y., S. Wang, X. Lu, H. Zhang, Y. Fu, and Y. Luo. Cholesterol sequestration by nystatin enhances the uptake and activity of endostatin in endothelium via regulating distinct endocytic pathways. Blood. 117:6392, 2011.
Cho, E. C., J. W. Xie, P. A. Wurm, and Y. N. Xia. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with I2/KI etchant. Nano Lett. 2009:9, 1080.
Conner, S. D., and S. L. Schmid. Regulated portals of entry into the cell. Nature. 422:37, 2003.
Crown, J., J. O’Shaughnessy, and G. Gullo. Emerging targeted therapies in triple-negative breast cancer. Ann. Oncol. 23(vi5):6, 2012.
Davis, M. E., Z. G. Chen, and D. M. Shin. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 7:771, 2008.
Doherty, G. J., and H. T. McMahon. Mechanisms of endocytosis. Ann. Rev. Biochem. 78:857, 2009.
Elhissi, A. M. A., W. Ahmed, I. U. Hassan, V. R. Dhanak, and A. D’Emanuele. Carbon nanotubes in cancer therapy and drug delivery. J. Drug Deliv. 2012:837327, 2012.
Farokhzad, O. C., and R. Langer. Impact of nanotechnology on drug delivery. ACS Nano. 3(1):16, 2009.
Ferrari, M. Actin reorganization contributes to loss of cell adhesion in pemphigus vulgaris. Nat. Rev. Cancer. 5:161, 2005.
Gliem, M., W.-M. Heupel, V. Spindler, G. S. Harms, and J. Waschke. Actin reorganization contributes to loss of cell adhesion in pemphigus vulgaris. Am. J. Physiol. 299(3):606, 2010.
He, H., L. A. Pham-Huy, P. Dramou, D. Xiao, P. Zuo, and C. Pham-Huy. Carbon nanotubes: applications in pharmacy and medicine. BioMed Res. Int., 2013
Ivanov, A. I. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol. Biol. 440:15, 2008.
Kim, J. S., T. J. Yoon, K. N. Yu, M. S. Noh, M. Woo, B. G. Kim, K. H. Lee, B. H. Sohn, S. B. Park, J. K. Lee, and M. H. Cho. Selective targeting of gold nanorods at the mitochondria of cancer cells: implications of cancer therapy. J. Vet. Sci. 11:772, 2006.
Kirchhausen, T., E. Macia, and H. E. Pelish. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 438:77, 2008.
Kostarelos, K., L. Lacerda, G. Pastorin, W. Wi, S. Wieckowski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J. P. Briand, S. Muller, M. Prato, and A. Bianco. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2:108, 2007.
Kumari, S., M. G. Swetha, and S. Mayor. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 20:256, 2010.
Liang, M., I. C. Lin, M. R. Whittaker, R. F. Minchin, M. J. Monteiro, and I. Toth. Cellular uptake of densely packed polymer coatings on gold nanoparticles. ACS Nano. 4:403, 2010.
Liu, Z., and X. J. Liang. Nano-carbons as theranostics. Theranostic 2(3):235, 2012.
Lundqvist, M., J. Stigler, G. Elia, I. Lynch, T. Cedervall, and A. K. Dawson. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 105:14265, 2008.
Macia, E., M. Ehrlich, R. Massol, E. Boucrot, C. Bruneer, and T. Kirchhausen. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell. 10(6):839, 2006.
Madani, S. Y., N. Naderi, O. Dissanayake, A. Tan, and A. M. Seifalian. A new era of cancer treatmemt: carbon nanotubes as drug delivery tools. Int. J. Nanomed. 6:2963–2979, 2011.
Matsumura, Y., and H. Maeda. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agents smancs. Cancer Res. 46:6387, 1986.
Mc Mahon, H. T., and E. Boucrot. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12:517, 2011.
Michael, M. D., B. C. Christopher, B. Jessica, S. Kelly, W. Linda, S. K. Gary, F. Vita, G. David, G. Robert, and H. Chris. Optimized high-throughtput microRNA expression profiling provides novel biomarker assessment of clinical prostrate and breast cancer biopsies. Mol. Cancer 5:24, 2006.
Misra, S. K., H.-H. Chang, P. Mukherjee, S. Tiwari, A. Ohoka, and D. Pan. Regulating biocompatibility of carbon spheres via defined nanoscale chemistry and a careful selection of surface functionalites. Sci. Rep. 5:14986, 2015.
Misra, S. K., J. Kus, S. Kim, and D. Pan. Nanoscopic poly-DNA-cleaver for breast cancer regression with induced oxidative damage. Mol. Pharm. 2014:33, 1976.
Misra, S. K., P. Mukherjee, H. H. Chang, S. Tiwari, M. Gryka, R. Bhargava, and D. Pan. Multi-functionality redefined with colloidal carotene carbon nanoparticles for synchronized chemical imaging, enriched cellular uptake and therapy. Sci Rep. 6:29299, 2016.
Misra, S. K., F. Ostadhossein, E. Daza, E. V. Johnson, and D. Pan. Hyperspectral imaging offers visual and quantitative evidence of drug release from Zwitterionic-Phospholipid-Nanocarbon when concurrently tracked in 3D intracellular space. Adv. Funct. Mater. 26:8031, 2016.
Misra, S. K., I. Srivastava, I. Tripathi, E. Daza, F. Ostadhossein, and D. Pan. Macromolecularly “caged” carbon nanoparticle for intracellular trafficking via switchable photoluminescence. J. Am. Chem. Soc. 139(5):1746–1749, 2017.
Misra, S. K., X. Wang, I. Srivastava, M. K. Imgruet, R. W. Graff, A. Ohoka, T. L. Kampert, H. Gao, and D. Pan. Combinatorial therapy for triple negative breast cancer using hyperstar polymer-based nanoparticles. Chem. Commun. 51:16710, 2005.
Misra, S. K., M. Ye, S. Kim, and D. Pan. Highly efficient anti-cancer therapy using scorpion ‘NanoVenin’. Chem. Commun. 50:13220, 2014.
Mukherjee, P., S. K. Misra, M. C. Gryka, H.-H. Chang, S. Tiwari, W. L. Wilson, J. W. Scott, R. Bhargava, and D. Pan. Tunable luminescent carbon nanospheres with well-defined nanoscale chemistry for synchronized imaging and therapy. Small 36:4691, 2016.
Ostadhossein, F., and D. Pan. Functional carbon nanodots for multiscale imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 9(3):e1436, 2017. doi:10.1002/wnan.1436.
Papakonstanti, E. A., and C. Stournaras. Cell responses regulated by early reorganization of actin cytoskeleton. FEBS Lett. 582(14):2120, 2008.
Qin, W., D. Ding, J. Liu, W. Z. Yuan, Y. Hu, B. Liu, and B. Z. Tang. Biocompatible nanoparticles with aggregation-induced emission characteristics as far-red/near-infrared fluorescent bioprobes for in vitro and in vivo imaging applications. Adv. Funct. Mater. 22(4):771, 2011.
Rakha, E. A., M. E. El-Sayed, A. R. Green, A. H. S. Lee, J. F. Robertson, and I. O. Ellis. Prognostic markers in triple-negative breast cancer. Cancer 109:25, 2007.
Ren, X., L. Duan, Q. He, Z. Zhang, Y. Zhou, D. Wu, J. Pan, D. Pei, and K. Ding. Identification of niclosamide as a new small-molecule inhibitor of the STAT3 signalling pathway. ACS Med. Chem. Lett. 1:454, 2010.
Saha, K., S. T. Kim, B. Yan, O. R. Miranda, F. S. Alfonso, D. Shlosman, and V. M. Rotello. Surface functionality of nanoparticles determines cellular uptake mechanisms in mammalian cells. Small. 9(2):300, 2013.
Sigismund, J. S., T. Woelk, C. Puri, E. Maspero, C. Tacchetti, P. Transidico, P. P. DiFiore, and S. Polo. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102:2760, 2005.
Srivastava, I., S. K. Misra, F. Ostadhossein, E. Daza, J. Singh, and D. Pan. Surface chemistry of carbon nanoparticles functionally select their uptake in various stages of cancer cells. Nano Res. 2017. doi:10.1007/s12274-017-1518-2.
Stuart, A. D., and T. D. K. Brown. Entry of feline calicivirus is dependent on clathrin-mediated endocytosis and acidification in endosomes. J. Cell Virol. 80(15):7500, 2006.
Swanson, J. A., and C. Watts. Macropinocytosis. Trends Cell Biol. 5:424, 1995.
Vercauteren, D., R. E. Vandenbroucke, A. T. Jones, J. Rejman, J. Demeester, S. C. DeSmedt, N. N. Sanders, and K. Braeckmans. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol. Therapy. 18(3):561, 2010.
Walkey, C. D., J. B. Olsen, H. Guo, A. Emill, and W. C. W. Chan. Nanoparticle size and surface chemistry determine serum proteins adsorption and macrophage uptake. J. Am. Chem. Soc. 134(4):2139, 2012.
Wang, T., J. Bai, X. Jiang, and G. U. Nienhaus. Cellular uptake of nanoparticles by membrane penetration: a study combining confocal microscopy with FTIR spectroelectrochemistry. ACS Nano. 6(2):1251, 2012.
Wang, Y.-C., T.-K. Chao, C.-C. Chang, Y.-T. Yo, M.-H. Yu, and H.-C. Lai. Drug screening identifies niclosamide as an inhibitor of breast cancer stem-like cells. PLoS ONE 8:74538, 2013.
Wang, L., Y. Liu, W. Li, X. Jiang, Y. Ji, X. Wu, L. Xu, Y. Qiu, K. Zhao, T. Wei, Y. Li, Y. Zhao, and C. Chen. Selective targeting of gold nanorods at the mitochondria of cancer cells: implications of cancer therapy. Nano Lett. 11(2):772, 2011.
Wang, L. H., K. G. Rothberg, and R. G. Anderson. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123(5):1107, 1993.
Xin-Sheng, D., W. Shuiliang, D. Anlong, L. Bolin, E. M. Susan, L. E. Stuart, W.-A. Reema, and T. D. Ann. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Ann. Cell Cycle 11:367, 2012.
Ye, T., Y. Xiong, Y. Yan, Y. Xia, X. Song, L. Liu, D. Li, N. Wang, L. Zhang, Y. Zhu, J. Zeng, Y. Wei, and L. Yu. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS ONE 9:P1, 2014.
Youan, B. B. Impact of nanoscience and nanotechnology on controlled drug delivery. Nanomedicine. 3(4):401, 2008.
Zhang, S. L., J. Li, G. Lykotrafitis, G. Bao, and S. Suresh. Size-dependent endocytosis of nanoparticles. Adv. Mater. 21:419, 2009.
Acknowledgments
Materials characterizations were done at Frederick Seitz Materials Research Laboratory, UIUC. We would like to thank Fatemeh Ostadhossein and Enrique Daza for help with the biodegradability studies.
Funding
Funding from UIUC, National Science Foundation, Michael Reese Foundation and Children’s Discovery Institute are acknowledged.
Conflict of Interest
Prof. Pan has received research grants from NIH, NSF, American Heart Association, Children’s Discovery Institute, Michael Reese Foundation and other agencies. Prof. Pan is the founder or co-founder of three start-up companies. None of these entities supported this research. Taylor Kampert, Indrajit Srivastava and Santosh Misra declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Prof. Dipanjan Pan, MS, PhD, is a recognized expert in nanomedicine, molecular imaging and drug delivery. He is an Associate Professor in Bioengineering and Materials Science and Engineering and Institute of Sustainability in Energy and Environment in University of Illinois, Urbana-Champaign. He holds a full faculty position with Beckman Institute for Advanced Science and Technology, University of Illinois Cancer Center and Carle Foundation Hospital. Administratively he directs the Professional Masters in Engineering Program in Bioengineering in the College of Engineering. He is also a course director for the newly founded engineering inspired medical school at the University of Illinois. Prior to coming to Illinois, he was an Assistant Professor in Medicine, Research in Washington University School of Medicine, St Louis. His research is highly collaborative and interdisciplinary centering on the development of novel materials for biomedical applications and targeted therapies for stem-like cancer cell and phenotypically screened nanomedicine platforms. Over the years, this research has resulted in more than 100 high impact peer reviewed publications in scientific journals, numerous conference abstracts and has been supported by external funding from NIH, NSF, DoD, American Heart Association and other sources. Prof. Pan edited and co-written two books published from Taylor and Francis (Nanomedicine: A Soft Matter Perspective, ISBN-13: 978-1466572829) and Springer (Personalized Medicine with a Nanochemistry Twist: Nanomedicine (Topics in Medicinal Chemistry, ISBN-13: 978-3319335445). He holds multiple patents, several ongoing clinical trials and is the founder of three University based early start-ups. He is the CEO/President for a biotechnology start-up Vitruvian Biotech dedicated to develop novel image guided therapies. He also co-founded InnSight Technologies dedicated to nanotechnology based application for ocular diseases. His other company Kalocyte, which he cofounded with his clinical collaborators, develops oxygen therapeutics. Technology developed in his laboratories has been licensed for commercial development multiple times. He serves frequently as study section review board member for NIH, CDMRP (DoD) and multiple review committee member for American Heart Association. In 2016 he received Nano-Micro Letter (NML) Researcher award. He is an elected fellow of Royal Society of Chemistry, and a Fellow of American Heart Association. Professor Pan is an editorial board member of Scientific Reports (Nature Publishing) and an editorial advisory board member of Molecular Pharmaceutics (ACS)

.
This article is part of the 2017 CMBE Young Innovators special issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kampert, T., Misra, S.K., Srivastava, I. et al. Phenotypically Screened Carbon Nanoparticles for Enhanced Combinatorial Therapy in Triple Negative Breast Cancer. Cel. Mol. Bioeng. 10, 371–386 (2017). https://doi.org/10.1007/s12195-017-0490-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-017-0490-y