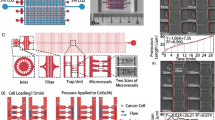
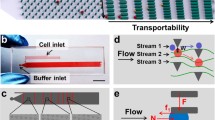
Abstract
Introduction
Circulating tumor cells (CTCs) in microcirculation undergo significant deformation and frictional interactions within microcapillaries. To understand the physical parameters governing their flow-induced transport, we studied the pressure-driven flow of cancer cells in a microfluidic model of a capillary network.
Methods
Our microfluidic device contains an array of parallel constrictions separated by regions where cells can repetitively deform and relax. To characterize the transport behavior, we measured the entry time, transit time, and shape deformation of tumor cells as they squeeze through the network.
Results
We found that entry and transit times of cells are much lower after repetitive deformation as their elongated shape enables easy transport in subsequent constrictions. Furthermore, upon repetitive deformation, the cells were able to relieve only 25% of their 40% imposed compressional strain, suggesting that tumor cells might have undergone plastic deformation or fatigue. To investigate the influence of surface friction, we characterized the transport behavior in the absence and presence of bovine serum albumin (BSA) coating on the constriction walls. We observed that BSA coating reduces the entry and transit time significantly. Finally, using two breast tumor cell lines, we investigated the effect of metastatic potential on transport properties. We found that the cell lines could be distinguished only upon surface treatment with BSA, thus surface-induced friction is an indicator of metastatic potential.
Conclusions
Our results suggest that pre-deformation can enhance the transport of CTCs in microcirculation and that frictional interactions with capillary walls can play an important role in influencing the transport of metastatic CTCs.







Similar content being viewed by others
References
Adamo, A., et al. Microfluidics-based assessment of cell deformability. Anal. Chem. 84:6438–6443, 2012.
Bhattacharya, S., A. Datta, J. M. Berg, and S. Gangopadhyay. Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 14:590–597, 2005.
Byun, S., et al. Characterizing deformability and surface friction of cancer cells. Proc. Natl Acad. Sci. USA 110:7580–7585, 2013.
Chambers, A. F., A. C. Groom, and I. C. MacDonald. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2:563–572, 2002.
Chang, Y. S., et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc. Natl Acad. Sci. USA 97:14608–14613, 2000.
Chen, J., et al. Classification of cell types using a microfluidic device for mechanical and electrical measurement on single cells. Lab Chip 11:3174–3181, 2011.
Cheng, Y. L., S. A. Darst, and C. R. Robertson. Bovine serum albumin adsorption and desorption rates on solid surfaces with varying surface properties. J. Colloid Interface Sci. 118:212–223, 1987.
Das, T., and S. Chakraborty. Perspective: flicking with flow: can microfluidics revolutionize the cancer research? Biomicrofluidics 7:11811, 2013.
di Tomaso, E., et al. Mosaic tumor vessels: cellular basis and ultrastructure of focal regions lacking endothelial cell markers. Cancer Res. 65:5740–5749, 2005.
Dobrzynska, I., E. Skrzydlewska, and Z. A. Figaszewski. Changes in electric properties of human breast cancer cells. J. Membr. Biol. 246:161–166, 2013.
Friedl, P., and K. Wolf. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer 3:362–374, 2003.
Fu, Y., L. K. Chin, T. Bourouina, A. Q. Liu, and A. M. VanDongen. Nuclear deformation during breast cancer cell transmigration. Lab Chip 12:3774–3778, 2012.
Gabriele, S., A. M. Benoliel, P. Bongrand, and O. Theodoly. Microfluidic investigation reveals distinct roles for actin cytoskeleton and myosin II activity in capillary leukocyte trafficking. Biophys. J. 96:4308–4318, 2009.
Gossett, D. R., et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl Acad. Sci. USA 109:7630–7635, 2012.
Guck, J., et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 88:3689–3698, 2005.
Guntheroth, W. G., D. L. Luchtel, and I. Kawabori. Pulmonary microcirculation: tubules rather than sheet and post. J. Appl. Physiol. 53:510–515, 1982.
Guofeng, G., et al. Real-time control of a microfluidic channel for size-independent deformability cytometry. J. Micromech. Microeng. 22:105037, 2012.
Guyton, A. C., and J. E. Hall. Textbook of Medical Physiology (11th ed.). Philadelphia: Elsevier Saunders, pp. 161–194, 2006.
Halldorsson, S., E. Lucumi, R. Gomez-Sjoberg, and R. M. Fleming. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 63:218–231, 2015.
Hillborg, H., et al. Crosslinked polydimethylsiloxane exposed to oxygen plasma studied by neutron reflectometry and other surface specific techniques. Polymer 41:6851–6863, 2000.
Hou, H. W., et al. Deformability study of breast cancer cells using microfluidics. Biomed. Microdevices 11:557–564, 2009.
Kamyabi, N., and S. A. Vanapalli. Microfluidic cell fragmentation for mechanical phenotyping of cancer cells. Biomicrofluidics 10:021102, 2016.
Kaneko, N., R. Matsuda, M. Toda, and K. Shimamoto. Three-dimensional reconstruction of the human capillary network and the intramyocardial micronecrosis. Am. J. Physiol. Heart Circ. Physiol. 300:H754–H761, 2011.
Khan, Z. S., N. Kamyabi, F. Hussain, and S. A. Vanapalli. Passage times and friction due to flow of confined cancer cells, drops, and deformable particles in a microfluidic channel. Converg. Sci. Phys. Oncol. 3:024001, 2017.
Khan, Z. S., and S. A. Vanapalli. Probing the mechanical properties of brain cancer cells using a microfluidic cell squeezer device. Biomicrofluidics 7:11806, 2013.
Krebs, M. G., et al. Molecular analysis of circulating tumour cells—biology and biomarkers. Nat. Rev. Clin. Oncol. 11:129–144, 2014.
Lange, J. R., et al. Microconstriction arrays for high-throughput quantitative measurements of cell mechanical properties. Biophys. J. 109:26–34, 2015.
Lee, J. N., X. Jiang, D. Ryan, and G. M. Whitesides. Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane). Langmuir 20:11684–11691, 2004.
Mak, M., and D. Erickson. A serial micropipette microfluidic device with applications to cancer cell repeated deformation studies. Integr. Biol. (Camb.) 5:1374–1384, 2013.
McDonald, D. M., and P. L. Choyke. Imaging of angiogenesis: from microscope to clinic. Nat. Med. 9:713–725, 2003.
Miyamoto, D. T., L. V. Sequist, and R. J. Lee. Circulating tumour cells—monitoring treatment response in prostate cancer. Nat. Rev. Clin. Oncol. 11:401–412, 2014.
Ng, J., Y. Shin, and S. Chung. Microfluidic platforms for the study of cancer metastasis. Biomed. Eng. Lett. 2:72–77, 2012.
Nguyen, D. X., P. D. Bos, and J. Massague. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9:274–284, 2009.
Nyberg, K. D., et al. The physical origins of transit time measurements for rapid, single cell mechanotyping. Lab Chip 16:3330–3339, 2016.
Otto, O., et al. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods 12:199–202, 2015.
Pantel, K., and M. R. Speicher. The biology of circulating tumor cells. Oncogene 2015. doi:10.1038/onc.2015.
Polacheck, W. J., R. Li, S. G. M. Uzel, and R. D. Kamm. Microfluidic platforms for mechanobiology. Lab Chip 13:2252–2267, 2013.
Preira, P., M.-P. Valignat, J. Bico, and O. Théodoly. Single cell rheometry with a microfluidic constriction: quantitative control of friction and fluid leaks between cell and channel walls. Biomicrofluidics 7:024111, 2013.
Ren, X., P. Ghassemi, H. Babahosseini, J. Strobl, and M. Agah. Single-cell mechanical characteristics analyzed by multi-constriction microfluidic channels. ACS Sens. 2017. doi:10.1021/acssensors.6b00823.
Ruths, M., A. D. Berman, and J. N. Israelachvili. In: Nanotribology and Nanomechanics: An Introduction, edited by B. Bhushan. Berlin: Springer, 2005, pp. 389–481.
Sakuma, S., et al. Red blood cell fatigue evaluation based on the close-encountering point between extensibility and recoverability. Lab Chip 14:1135–1141, 2014.
Schmidt, R. F., and G. Thews (eds.). Human Physiology (2nd ed.). Berlin: Springer, 1989.
Schrott, W., et al. Study on surface properties of PDMS microfluidic chips treated with albumin. Biomicrofluidics 3:44101, 2009.
Sobin, S. S., H. M. Tremer, and Y. C. Fung. Morphometric basis of the sheet-flow concept of the pulmonary alveolar microcirculation in the cat. Circ. Res. 26:397–414, 1970.
Tomaiuolo, G., et al. Microfluidics analysis of red blood cell membrane viscoelasticity. Lab Chip 11:449–454, 2011.
Turitto, V. T. Blood viscosity, mass transport, and thrombogenesis. Prog. Hemost. Thromb. 6:139–177, 1982.
Vanapalli, S. A., M. H. Duits, and F. Mugele. Microfluidics as a functional tool for cell mechanics. Biomicrofluidics 3:12006, 2009.
Weinbaum, S., S. C. Cowin, and Y. Zeng. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 27:339–360, 1994.
Weinbaum, S., Y. Duan, L. M. Satlin, T. Wang, and A. M. Weinstein. Mechanotransduction in the renal tubule. Am. J. Physiol. Renal Physiol. 299:F1220–F1236, 2010.
Williams, S. A., et al. Dynamic measurement of human capillary blood pressure. Clin. Sci. (Lond.) 74:507–512, 1988.
Wirtz, D., K. Konstantopoulos, and P. C. Searson. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11:512–522, 2011.
Xia, Y., and G. M. Whitesides. Soft lithography. Annu. Rev. Mater. Sci. 28:153–184, 1998.
Xue, C., et al. Constriction channel based single-cell mechanical property characterization. Micromachines 6:1457, 2015.
Yamauchi, K., et al. Real-time in vivo dual-color imaging of intracapillary cancer cell and nucleus deformation and migration. Cancer Res. 65:4246–4252, 2005.
Zhang, Z., and S. Nagrath. Microfluidics and cancer: are we there yet? Biomed. Microdevices 15:595–609, 2013.
Acknowledgments
We acknowledge support from the Cancer Prevention and Research Institute of Texas (Grant No. RP 140298).
Conflicts of interest
Nabiollah Kamyabi, Zeina S. Khan and Siva A. Vanapalli declare that they have no conflicts of interest.
Ethical Standards
No human studies or animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamyabi, N., Khan, Z.S. & Vanapalli, S.A. Flow-Induced Transport of Tumor Cells in a Microfluidic Capillary Network: Role of Friction and Repeated Deformation. Cel. Mol. Bioeng. 10, 563–576 (2017). https://doi.org/10.1007/s12195-017-0499-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-017-0499-2