Abstract
Introduction
Tumor metastasis to the brain occurs in approximately 20% of all cancer cases and often occurs due to tumor cells crossing the blood-brain barrier (BBB). The brain microenvironment is comprised of a soft hyaluronic acid (HA)-rich extracellular matrix with an elastic modulus of 0.1–1 kPa, whose crosslinking is often altered in disease states.
Methods
To explore the effects of HA crosslinking on breast tumor cell migration, we developed a biomimetic model of the human brain endothelium, consisting of brain microvascular endothelial cell (HBMEC) monolayers on HA and gelatin (HA/gelatin) films with different degrees of crosslinking, as established by varying the concentration of the crosslinker Extralink.
Results and Discussion
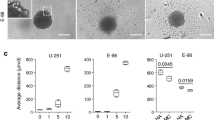
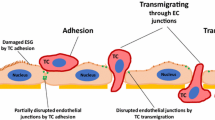
Metastatic breast tumor cell migration speed, diffusion coefficient, spreading area, and aspect ratio increased with decreasing HA crosslinking, a mechanosensing trend that correlated with tumor cell actin organization but not CD44 expression. Meanwhile, breast tumor cell incorporation into endothelial monolayers was independent of HA crosslinking density, suggesting that alterations in HA crosslinking density affect tumor cells only after they exit the vasculature. Tumor cells appeared to exploit both the paracellular and transcellular routes of trans-endothelial migration. Quantitative phenotyping of HBMEC junctions via a novel Python software revealed a VEGF-dependent decrease in punctate VE-cadherin junctions and an increase in continuous and perpendicular junctions when HBMECs were treated with tumor cell-secreted factors.
Conclusions
Overall, our quantitative results suggest that a combination of biochemical and physical factors promote tumor cell migration through the BBB.










Similar content being viewed by others
Abbreviations
- AFM:
-
Atomic force microscopy
- ANOVA:
-
Analysis of variance
- AR:
-
Aspect ratio
- BBB:
-
Blood-brain barrier
- BSA:
-
Bovine serum albumin
- DMEM:
-
Dulbecco’s modified eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- ECGS:
-
Endothelial cell growth supplement
- ECM:
-
Extracellular matrix
- FBS:
-
Fetal bovine serum
- GFP:
-
Green fluorescent protein
- HA:
-
Hyaluronic acid
- HBMEC:
-
Human brain microvascular endothelial cell
- JanaP:
-
Junction Analyzer Program
- LOX:
-
Lysyl oxidase
- PBS:
-
Phosphate buffered saline
- RPMI:
-
Roswell Park Memorial Institute
- STR:
-
Short tandem repeat
- TCM:
-
Tumor conditioned media
- TJ:
-
Tight junctions
- VE-cadherin:
-
Vascular endothelial cadherin
- VEGF:
-
Vascular Endothelial Growth Factor
- ZO-1:
-
Zonula occludens-1
References
Abbott, N. J., and A. Friedman. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia 53(Suppl 6):1–6, 2012.
Achrol, A. S., R. C. Rennert, C. Anders, R. Soffietti, M. S. Ahluwalia, L. Nayak, S. Peters, N. D. Arvold, G. R. Harsh, P. S. Steeg, and S. D. Chang. Brain metastases. Nat. Rev. Dis. Primers 5:5, 2019.
Ajami, N. E., S. Gupta, M. R. Maurya, P. Nguyen, J. Y.-S. Li, J. Y.-J. Shyy, Z. Chen, S. Chien, and S. Subramaniam. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc. Natl. Acad. Sci. 114:10990–10995, 2017.
Akiri, G., E. Sabo, H. Dafni, Z. Vadasz, Y. Kartvelishvily, N. Gan, O. Kessler, T. Cohen, M. Resnick, M. Neeman, and G. Neufeld. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 63:1657–1666, 2003.
Ananthanarayanan, B., Y. Kim, and S. Kumar. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials 32:7913–7923, 2011.
Arshad, F., L. Wang, C. Sy, S. Avraham, and H. K. Avraham. Blood-brain barrier integrity and breast cancer metastasis to the brain. Patholog. Res. Int. 1–12:2010, 2011.
Arvanitis, C., S. Khuon, R. Spann, K. M. Ridge, T.-L. Chew, and L. Kreplak. Structure and biomechanics of the endothelial transcellular circumferential invasion array in tumor invasion. PLoS ONE 9:e89758, 2014.
Avraham, H. K., S. Jiang, Y. Fu, H. Nakshatri, H. Ovadia, and S. Avraham. Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J. Pathol. 232:369–381, 2014.
Baeyens, N., C. Bandyopadhyay, B. G. Coon, S. Yun, and M. A. Schwartz. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Invest. 126:821–828, 2016.
Barnes, J. M., L. Przybyla, and V. M. Weaver. Tissue mechanics regulate brain development, homeostasis and disease. J. Cell Sci. 130:71–82, 2017.
Bellail, A. C., S. B. Hunter, D. J. Brat, and E. G. Van Meir. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int. J. Biochem. Cell Biol. 36:1046–1069, 2004.
Cai, J., W. G. Jiang, and R. E. Mansel. Phosphorylation and disorganization of vascular-endothelial cadherin in interaction between breast cancer and vascular endothelial cells. Int. J. Mol. Med. 4:191–195, 1999.
Chen, W., A. D. Hoffmann, H. Liu, and X. Liu. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. Precis. Oncol. 2:4, 2018.
Cox, T. R., and J. T. Erler. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model. Mech. 4:165–178, 2011.
Destefano, J. G., J. J. Jamieson, R. M. Linville, and P. C. Searson. Benchmarking in vitro tissue-engineered blood-brain barrier models. Fluids Barriers CNS 15:32, 2018.
DeStefano, J. G., Z. S. Xu, A. J. Williams, N. Yimam, and P. C. Searson. Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs). Fluids Barriers CNS 14:20, 2017.
Discher, D. E., P. Janmey, and Y.-L. Wang. Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143, 2005.
Dorland, Y. L., and S. Huveneers. Cell-cell junctional mechanotransduction in endothelial remodeling. Cell. Mol. Life Sci. 74:279–292, 2017.
Dun, M. D., R. J. Chalkley, S. Faulkner, S. Keene, K. A. Avery-Kiejda, R. J. Scott, L. G. Falkenby, M. J. Cairns, M. R. Larsen, R. A. Bradshaw, and H. Hondermarck. Proteotranscriptomic profiling of 231-BR breast cancer cells: identification of potential biomarkers and therapeutic targets for brain metastasis. Mol. Cell. Proteomics 14:2316–2330, 2015.
Eddy, R. J., M. D. Weidmann, V. P. Sharma, and J. S. Condeelis. Tumor cell invadopodia: invasive protrusions that orchestrate metastasis. Trends Cell Biol. 27:595–607, 2017.
Eichler, A. F., E. Chung, D. P. Kodack, J. S. Loeffler, D. Fukumura, and R. K. Jain. The biology of brain metastases-translation to new therapies. Nat. Rev. Clin. Oncol. 8:344–356, 2011.
Fan, J., and B. M. Fu. Quantification of malignant breast cancer cell MDA-MB-231 transmigration across brain and lung microvascular endothelium. Ann. Biomed. Eng. 44:2189–2201, 2016.
Fazakas, C., I. Wilhelm, P. Nagyoszi, A. E. Farkas, J. Haskó, J. Molnar, H. Bauer, H.-C. Bauer, F. Ayaydin, N. T. K. Dung, L. Siklós, and I. A. Krizbai. Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases. PLoS ONE 6:e20758, 2011.
Grammas, P., J. Martinez, and B. Miller. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev. Mol. Med. 13:e19, 2011.
Gray, K. M., D. B. Katz, E. G. Brown, and K. M. Stroka. Quantitative phenotyping of cell-cell junctions to evaluate ZO-1 presentation in brain endothelial cells. Ann. Biomed. Eng. 2019. https://doi.org/10.1007/s10439-019-02266-5.
Hagedorn, E. J., J. W. Ziel, M. A. Morrissey, L. M. Linden, Z. Wang, Q. Chi, S. A. Johnson, and D. R. Sherwood. The netrin receptor DCC focuses invadopodia-driven basement membrane transmigration in vivo. J. Cell Biol. 201:903–913, 2013.
Hamilla, S. M., K. M. Stroka, and H. Aranda-Espinoza. VE-Cadherin-independent cancer cell incorporation into the vascular endothelium precedes transmigration. PLoS ONE 9:e109748, 2014.
Hielscher, A., K. Ellis, C. Qiu, J. Porterfield, and S. Gerecht. Fibronectin deposition participates in extracellular matrix assembly and vascular morphogenesis. PLoS ONE 11:e0147600, 2016.
Hoshino, A., et al. Tumour exosome integrins determine organotropic metastasis. Nature 527:329–335, 2015.
Jamieson, J. J., P. C. Searson, and S. Gerecht. Engineering the human blood-brain barrier in vitro. J. Biol. Eng. 11:37, 2017.
Kass, L., J. T. Erler, M. Dembo, and V. M. Weaver. Mammary epithelial cell: Influence of extracellular matrix composition and organization during development and tumorigenesis. Int. J. Biochem. Cell Biol. 3(39):1987–1994, 2007.
Katt, M. E., R. M. Linville, L. N. Mayo, Z. S. Xu, and P. C. Searson. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: the role of matrix composition on monolayer formation. Fluids Barriers CNS 15:7, 2018.
Kienast, Y., L. Von Baumgarten, M. Fuhrmann, W. E. F. Klinkert, R. Goldbrunner, J. Herms, and F. Winkler. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16:116–122, 2010.
Kim, Y., and S. Kumar. CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol. Cancer Res. 12:1416–1429, 2014.
Kohn, J. C. C., D. W. W. Zhou, F. Bordeleau, A. L. L. Zhou, B. N. N. Mason, M. J. J. Mitchell, M. R. R. King, and C. A. A. Reinhart-King. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys. J. 108:471–478, 2015.
Lee, H. J., M. F. Diaz, K. M. Price, J. A. Ozuna, S. Zhang, E. M. Sevick-Muraca, J. P. Hagan, and P. L. Wenzel. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 8:14122, 2017.
Lee, T.-H., H. Karsenty Avraham, S. Jiang, and S. Avraham. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J. Biol. Chem. 278:5277–5284, 2003.
Lee, K. Y., Y.-J. Kim, H. Yoo, S. H. Lee, J. B. Park, and H. J. Kim. Human brain endothelial cell-derived COX-2 facilitates extravasation of breast cancer cells across the blood-brain barrier. Anticancer Res. 31:4307–4313, 2011.
Leong, H. S., A. E. Robertson, K. Stoletov, S. J. Leith, C. A. Chin, A. E. Chien, M. N. Hague, A. Ablack, K. Carmine-Simmen, V. A. Mcpherson, C. O. Postenka, E. A. Turley, S. A. Courtneidge, A. F. Chambers, and J. D. Lewis. Article invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 8:1558–1570, 2014.
Levental, K. R., H. Yu, L. Kass, J. N. Lakins, M. Egeblad, J. T. Erler, S. F. T. Fong, K. Csiszar, A. Giaccia, W. Weninger, M. Yamauchi, D. L. Gasser, and V. M. Weaver. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906, 2009.
Li, B., W.-D. Zhao, Z.-M. Tan, W.-G. Fang, L. Zhu, and Y.-H. Chen. Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett. 580:4252–4260, 2006.
Mader, C. C., M. Oser, M. A. O. Magalhaes, J. J. Bravo-Cordero, J. Condeelis, A. J. Koleske, and H. Gil-Henn. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. J. Cancer Res. 71:OF1–OF12, 2011.
Martin, T. A., and W. G. Jiang. Loss of tight junction barrier function and its role in cancer metastasis. Biochim. Biophys. Acta Biomembr. 1788:872–891, 2009.
McFarlane, S., J. A. Coulter, P. Tibbits, A. O’Grady, C. McFarlane, N. Montgomery, A. Hill, H. O. McCarthy, L. S. Young, E. W. Kay, C. M. Isacke, and D. J. J. Waugh. CD44 increases the efficiency of distant metastasis of breast cancer. Oncotarget 6:11465–11476, 2015.
Mouw, J. K., G. Ou, and V. M. Weaver. Extracellular matrix assembly: a multiscale deconstruction. Nat. Publ. Gr. 15:771, 2014.
Narkhede, A. A., J. H. Crenshaw, R. M. Manning, and S. S. Rao. The influence of matrix stiffness on the behavior of brain metastatic breast cancer cells in a biomimetic hyaluronic acid hydrogel platform. J. Biomed. Mater. Res. A 106:1832–1841, 2018.
Nayak, L., E. Q. Lee, and P. Y. Wen. Epidemiology of brain metastases. Curr. Oncol. Rep. 14:48–54, 2012.
Northcott, J. M., I. S. Dean, J. K. Mouw, and V. M. Weaver. Feeling stress: the mechanics of cancer progression and aggression. Front. Cell Dev. Biol. 6:17, 2018.
Novak, U., and A. H. Kaye. Extracellular matrix and the brain: components and function. J. Clin. Neurosci. 7:280–290, 2000.
Onken, M. D., J. Li, and J. A. Cooper. Uveal melanoma cells utilize a novel Route for transendothelial migration. PLoS ONE 9:e115472, 2014.
Onken, M. D., O. L. Mooren, S. Mukherjee, S. T. Shahan, J. Li, and J. A. Cooper. Endothelial monolayers and transendothelial migration depend on mechanical properties of the substrate. Cytoskeleton 71:695–706, 2014.
Pogoda, K., R. Bucki, F. J. Byfield, K. Cruz, T. Lee, C. Marcinkiewicz, and P. A. Janmey. Soft substrates containing hyaluronan mimic the effects of increased stiffness on morphology, motility, and proliferation of glioma cells. Biomacromolecules 18:3040–3051, 2017.
Prestwich, G. D., and C. O. N. Spectus. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc. Chem. Res. 41:139–148, 2008.
Reymond, N., P. Riou, and A. J. Ridley. Rho GTPases and cancer cell transendothelial migration. Methods Mol. Biol. 827:123–142, 2012.
Roberts, H. C., T. P. L. Roberts, R. C. Brasch, and W. P. Dillon. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced mr imaging: correlation with histologic grade. AJNR Am. J. Neuroradiol. 21:891–899, 2000.
Rodriguez, P. L., S. Jiang, Y. Fu, S. Avraham, and H. K. Avraham. The proinflammatory peptide substance P promotes blood-brain barrier breaching by breast cancer cells through changes in microvascular endothelial cell tight junctions. Int. J. Cancer 134:1034–1044, 2014.
Roh-Johnson, M., J. J. Bravo-Cordero, A. Patsialou, V. P. Sharma, P. Guo, H. Liu, L. Hodgson, and J. Condeelis. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33:4203–4212, 2014.
Sarrió, D., S. M. Rodriguez-Pinilla, D. Hardisson, A. Cano, G. Moreno-Bueno, and J. Palacios. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 68:989–997, 2008.
Shaw, S. K., P. S. Bamba, B. N. Perkins, and F. W. Luscinskas. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 167:2323–2330, 2001.
Shumakovich, M. A., C. P. Mencio, J. S. Siglin, R. A. Moriarty, H. M. Geller, and K. M. Stroka. Astrocytes from the brain microenvironment alter migration and morphology of metastatic breast cancer cells. FASEB J. 31:5049–5067, 2017.
Sibony-Benyamini, H., and H. Gil-Henn. Invadopodia: the leading force. Eur. J. Cell Biol. 91:896–901, 2012.
Stroka, K. M., and H. Aranda-Espinoza. Neutrophils display biphasic relationship between migration and substrate stiffness. Cell Motil. Cytoskelet. 66:328–341, 2009.
Stroka, K. M., H. N. Hayenga, and H. Aranda-Espinoza. Human neutrophil cytoskeletal dynamics and contractility actively contribute to trans-endothelial migration. PLoS ONE 8:61377, 2013.
Stroka, K. M., B. Sheng Wong, M. Shriver, J. M. Phillip, D. Wirtz, A. Kontrogianni-Konstantopoulos, and K. Konstantopoulos. Loss of giant obscurins alters breast epithelial cell mechanosensing of matrix stiffness. Oncotarget 5:54004–54020, 2016.
Tornavaca, O., M. Chia, N. Dufton, L. O. Almagro, D. E. Conway, A. M. Randi, M. A. Schwartz, K. Matter, and M. S. Balda. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 208:821–838, 2015.
Turitto, V. T. Blood viscosity, mass transport, and thrombogenesis. Prog. Hemost. Thromb. 6:139–177, 1982.
Vallenius, T. Actin stress fibre subtypes in mesenchymal-migrating cells. Open Biol. 3:130001, 2013.
Vanderhooft, J. L., M. Alcoutlabi, J. J. Magda, and G. D. Prestwich. Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromol. Biosci. 2009. https://doi.org/10.1002/mabi.200800141.
Wrobel, J. K., and M. Toborek. Blood–brain barrier remodeling during brain metastasis formation. Mol. Med. 22:32–40, 2016.
Yankaskas, C. L., K. N. Thompson, C. D. Paul, M. I. Vitolo, P. Mistriotis, A. Mahendra, V. K. Bajpai, D. J. Shea, K. M. Manto, A. C. Chai, N. Varadarajan, A. Kontrogianni-Konstantopoulos, S. S. Martin, and K. Konstantopoulos. A microfluidic assay for the quantification of the metastatic propensity of breast cancer specimens. Nat. Biomed. Eng. 2019. https://doi.org/10.1038/s41551-019-0400-9.
Ye, M., H. M. Sanchez, M. Hultz, Z. Yang, M. Bogorad, A. D. Wong, and P. C. Searson. Brain microvascular endothelial cells resist elongation due to curvature and shear stress. Sci. Rep. 4:4681, 2014.
Yeung, T., P. C. Georges, L. A. Flanagan, B. Marg, M. Ortiz, M. Funaki, N. Zahir, W. Ming, V. Weaver, and P. A. Janmey. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 60:24–34, 2005.
Yoneda, T., P. J. Williams, T. Hiraga, M. Niewolna, and R. Nishimura. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J. Bone Miner. Res. 16:1486–1495, 2001.
Zhang, P., C. Fu, H. Bai, E. Song, and Y. Song. CD44 variant, but not standard CD44 isoforms, mediate disassembly of endothelial VE-cadherin junction on metastatic melanoma cells. FEBS Lett. 588:4573–4582, 2014.
Zheng Shu, X., Y. Liu, F. S. Palumbo, Y. Luo, and G. D. Prestwich. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 25:1339–1348, 2004.
Acknowledgments
We thank Dr. Toshiyuki Yoneda for generously providing MDA-MB-231-BR cells. The University of Maryland Computer, Mathematical, and Natural Sciences imaging incubator is acknowledged for providing training and equipment for confocal imaging. Kyle Thomas at Yellow Basket, LLC (kyle@yellowbasket.io) is acknowledged for the JAnaP software development support. We also acknowledge Mary Doolin for help with editing custom Matlab code. We thank Dr. William Luscinskas from the Harvard Medical School for generously providing us with the VE-cadherin-GFP adenovirus.
Funding
Funding was provided by Burroughs Wellcome Fund (Career Award at the Scientific Interface). Additional funding was provided by the Ann G. Wylie Dissertation Fellowship from the University of Maryland Graduate School (to MAP), the Fischell Fellowship in Biomedical Engineering (to KMG), the Dr. Mabel S. Spencer Award for Excellence in Graduate Achievement (to KMG), the Clark Doctoral Fellowship (to AJD), the Fischell Department of Bioengineering, and the University of Maryland.
Author Contributions
KMS, MAP, and KMG designed the research. MAP and GMD performed experiments for Fig. 1. GMD analyzed all data for Fig. 1. AJLD performed all experiments and data analysis for Fig. 2, with guidance from MAP. MAP performed confocal microscopy for Fig. 3. KMG performed experiments and analysis for Figs. 4, 5, 6, S2, S3, and S4, with help in analysis from JWJ. KMG prepared Fig. S1. MAP performed experiments and analysis for Figs. 7a–7e. AJLD performed experiments for Figs. 7f–7h, with guidance from MAP, and MAP analyzed data for Figs. 7f–7h. MAP performed confocal microscopy for Fig. 8. MAP and AJLD performed experiments for Fig. 9. MAP performed experiments and all analysis for Fig. 10. MAP performed statistical analysis for Figs. 1, 2, 7, and 10. MAP formatted Figs. 1, 2, 3, 7, 8, 9, and 10. KMG performed statistical analysis and/or formatting for Figs. 4, 5, 6, S2, S3, and S4. MAP, KMG, and KMS wrote the manuscript. All authors edited the manuscript, and all authors reviewed and approved final version of the manuscript.
Conflict of interest
MAP, KMG, GMD, AJLD, JWJ, and KMS declare that they have no conflict of interest.
Human Studies
No human studies were carried out by the authors for this article.
Animal Studies
No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kimberly Stroka, in January 2015, joined the Fischell Department of Bioengineering as an Assistant Professor at the University of Maryland, College Park. She received her B.S. in Physics in 2006 from Denison University and her PhD in Bioengineering from the University of Maryland, College Park. She completed her postdoctoral training at Johns Hopkins University in the Department of Chemical and Biomolecular Engineering and Institute for NanoBioTechnology. Dr. Stroka has received the National Science Foundation Graduate Research Fellowship, NIH NRSA F31 predoctoral fellowship, NIH T32 and F32 postdoctoral fellowships, and Burroughs Wellcome Career Award at the Scientific Interface. She also received the Rita Schaffer Young Investigator Award from the Biomedical Engineering Society (2014), Research and Scholarship Award from the UMD Graduate School (2017), “Outstanding Young Scientist Award” from the Maryland Academy of Sciences (2017), and Fischell Department of Bioengineering Faculty Teaching Award (2018). Dr. Stroka’s lab engineers cells and their microenvironment in order to create model systems that allow them to systematically understand fundamental aspects of cellular and tissue mechanobiology, with applications in vascular biomechanics, tumor cell metastasis, and stem cell engineering.

This article is part of the CMBE 2019 Young Innovators special issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pranda, M.A., Gray, K.M., DeCastro, A.J.L. et al. Tumor Cell Mechanosensing During Incorporation into the Brain Microvascular Endothelium. Cel. Mol. Bioeng. 12, 455–480 (2019). https://doi.org/10.1007/s12195-019-00591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-019-00591-2