Abstract
Introduction
In vivo, breast cancer cells spend on average 3–7 days adhered to the endothelial cells inside the vascular lumen before entering the brain. IL-1β is one of the highly upregulated molecules in brain-seeking triple negative breast cancer (TNBC) cells. In this study, the effect of IL-1β on the blood–brain barrier (BBB) and astrocytes and its role in transmigration of TNBC cells were evaluated.
Methods
The effect of IL-1β on transendothelial electrical resistance, gene and protein expression of human induced pluripotent stem cell-derived brain-specific microvascular endothelial-like cells (iBMECs) was studied. Transport of IL-1β across the iBMEC layer was investigated and the effect of IL-1β treatment of astrocytes on their cytokine and chemokine secretome was evaluated with a cytokine membrane array. Using BBB-on-a-chip devices, transmigration of MDA-MB-231 cells and their brain-seeking variant (231BR) across the iBMECs was studied, and the effect of an IL-1β neutralizing antibody on TNBC cell transmigration was investigated.
Results
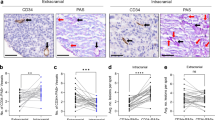
We showed that IL-1β reduces BBB integrity and induces endothelial-to-mesenchymal transition in iBMECs. IL-1β crosses the iBMEC layer and induces secretion of multiple chemokines by astrocytes, which can enhance TNBC cell transmigration across the BBB. Transmigration assays in a BBB-on-a-chip device showed that 231BR cells have a higher rate of transmigration across the iBMECs compared to MDA-MB-231 cells, and IL-1β pretreatment of BBB-on-a-chip devices increases the number of transmigrated MDA-MB-231 cells. Finally, we demonstrated that neutralizing IL-1β reduces the rate of 231BR cell transmigration.
Conclusion
IL-1β plays a significant role in transmigration of brain-seeking TNBC cells across the BBB.






Similar content being viewed by others
References
Al-Yafeai, Z., A. Yurdagul, J. M. Peretik, M. Alfaidi, P. A. Murphy, and A. W. Orr. Endothelial FN (Fibronectin) deposition by α5β1 integrins drives atherogenic inflammation. Arterioscler. Thromb. Vasc. Biol. 38:2601–2614, 2018.
Al-Yahya, S., et al. Human Cytokinome Analysis for Interferon Response. J. Virol. 89:7108–7119, 2015.
Amaral, M. L., G. A. Erikson, and M. N. Shokhirev. BART: bioinformatics array research tool. BMC Bioinformatics. 19:1–6, 2018.
An, G., et al. Effects of CCL5 on the biological behavior of breast cancer and the mechanisms of its interaction with tumor-associated macrophages. Oncol. Rep. 42:2499–2511, 2019.
Argaw, A. T., et al. IL-1β regulates blood–brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 177:5574–5584, 2006.
Avraham, H. K., S. Jiang, Y. Fu, H. Nakshatri, H. Ovadia, and S. Avraham. Angiopoietin-2 mediates blood–brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J. Pathol. 232:369–381, 2014.
Beard, R. S., et al. Non-muscle Mlck is required for ß-catenin- and FoxO1-dependent downregulation of Cldn5 in IL-1ß-mediated barrier dysfunction in brain endothelial cells. J. Cell Sci. 127:1840–1853, 2014.
Bersini, S., et al. A microfluidic 3D invitro model for specificity of breast cancer metastasis to bone. Biomaterials. 35:2454–2461, 2014.
Blamire, A. M., D. C. Anthony, B. Rajagopalan, N. R. Sibson, V. H. Perry, and P. Styles. Interleukin-1β-induced changes in blood–brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: A magnetic resonance study. J. Neurosci. 20:8153–8159, 2000.
Bos, P. D., et al. Genes that mediate breast cancer metastasis to the brain. Nature. 459:1005–1009, 2009.
Bubendorf, L., et al. Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum. Pathol. 31:578–583, 2000.
Chaudhuri, V., L. Zhou, and M. Karasek. Inflammatory cytokines induce the transformation of human dermal microvascular endothelial cells into myofibroblasts: a potential role in skin fibrogenesis. J. Cutan. Pathol. 34:146–153, 2007.
Deeb, A., S.-U. Haque, and O. Olowokure. Pulmonary metastases in pancreatic cancer, is there a survival influence? J. Gastrointest. Oncol. 6:E48-51, 2015.
Derada Troletti, C., et al. Inflammation-induced endothelial to mesenchymal transition promotes brain endothelial cell dysfunction and occurs during multiple sclerosis pathophysiology. Cell Death Dis. 10:1–13, 2019.
Drolez, A., et al. Selection of a relevant in vitro blood–brain barrier model to investigate Pro-Metastatic features of human breast cancer cell lines. PLoS ONE. 11:1–18, 2016.
Fan, J., and B. M. Fu. Quantification of malignant breast cancer cell MDA-MB-231 transmigration across brain and lung microvascular endothelium. Ann. Biomed. Eng. 44:2189–2201, 2016.
Fares, J., D. Kanojia, A. Rashidi, I. Ulasov, and M.S. Lesniak. Genes that mediate metastasis across the blood–brain barrier. Trends in Cancer 6:660–676, 2020.
Farmaki, E., I. Chatzistamou, V. Kaza, and H. Kiaris. A CCL8 gradient drives breast cancer cell dissemination. Physiol. Behav. 176:139–148, 2017.
Ferreira, F. U., et al. Endothelial cells tissue-specific origins affects their responsiveness to TGF-β2 during endothelial-to-mesenchymal transition. Int. J. Mol. Sci. 20:1–14, 2019.
Gasparics, Á., L. Rosivall, I. A. Krizbai, and A. Sebe. When the endothelium scores an own goal: endothelial cells actively augment metastatic extravasation through endothelial-mesenchymal transition. Am. J. Physiol. Heart Circ. Physiol. 310(9):H1055–H1063, 2016.
Gray, K. M., D. B. Katz, E. G. Brown, and K. M. Stroka. Quantitative phenotyping of cell–cell junctions to evaluate ZO-1 presentation in brain endothelial cells. Ann. Biomed. Eng. 47:1675–1687, 2019.
Hanahan, D., and R. A. Weinberg. Hallmarks of cancer: The next generation. Cell 144:646–674, 2011.
Harati, R., S. Hafezi, A. Mabondzo, and A. Tlili. Silencing miR-202-3p increases MMP-1 and promotes a brain invasive phenotype in metastatic breast cancer cells. PLoS ONE. 15:1–26, 2020.
Haskó, J., et al. Response of the neurovascular unit to brain metastatic breast cancer cells. Acta Neuropathol. Commun. 7:133, 2019.
Heitz, F., et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur. J. Cancer. 45:2792–2798, 2009.
Herman, H., et al. Paracellular and transcellular migration of metastatic cells through the cerebral endothelium. J. Cell. Mol. Med. 23:2619–2631, 2019.
Hewett, S. J., N. A. Jackman, and R. J. Claycomb. Interleukin-1β in Central Nervous System Injury and Repair. Eur. J. Neurodegener. Dis. 1:195–211, 2012.
Kemper, E. M., W. Boogerd, I. Thuis, J. H. Beijnen, and O. van Tellingen. Modulation of the blood–brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat. Rev. 30:415–423, 2004.
Kim, M. O., H. S. Suh, C. F. Brosnan, and S. C. Lee. Regulation of RANTES/CCL5 expression in human astrocytes by interleukin-1 and interferon-β. J. Neurochem. 90:297–308, 2004.
Klein, S., et al. α5β1 Integrin activates an NF-κB-dependent program of gene expression important for angiogenesis and inflammation. Mol. Cell. Biol. 22:5912–5922, 2002.
Krizbai, I. A., et al. Endothelial-mesenchymal transition of brain endothelial cells: possible role during metastatic extravasation. PLoS ONE. 10:1–19, 2015.
Lee, Y. T., and D. A. Geer. Primary liver cancer: pattern of metastasis. J. Surg. Oncol. 36:26–31, 1987.
Li, J. Y., et al. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Res. Treat. 131:837–848, 2012.
Lim, S. Y., A. E. Yuzhalin, A. N. Gordon-Weeks, and R. J. Muschel. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 7:28697–28710, 2016.
Lin, C.-C., and B. T. Edelson. New insights into the role of IL-1β in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Immunol. 198:4553–4560, 2017.
Lippmann, E. S., A. Al-Ahmad, S. M. Azarin, S. P. Palecek, and E. V. Shusta. A retinoic acid-enhanced, multicellular human blood–brain barrier model derived from stem cell sources. Sci. Rep. 4:4160, 2014.
Lippmann, E. S., S. M. Azarin, S. P. Palecek, and E. V. Shusta. Commentary on human pluripotent stem cell-based blood–brain barrier models. Fluids Barriers CNS BioMed Central. 17:4–9, 2020.
Lorger, M., and B. Felding-Habermann. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am. J. Pathol. 176:2958–2971, 2010.
Lorger, M., H. Lee, J. S. Forsyth, and B. Felding-Habermann. Comparison of in vitro and in vivo approaches to studying brain colonization by breast cancer cells. J. Neurooncol. 104:689–696, 2011.
Maleszewska, M., J. R. A. J. Moonen, N. Huijkman, B. van de Sluis, G. Krenning, and M. C. Harmsen. IL-1β and TGFβ2 synergistically induce endothelial to mesenchymal transition in an NFκB-dependent manner. Immunobiology. 218:443–454, 2013.
McLay, R. N., A. J. Kastin, and J. E. Zadina. Passage of interleukin-1-beta across the blood–brain barrier is reduced in aged mice: a possible mechanism for diminished fever in aging. Neuroimmunomodulation. 8:148–153, 2000.
Miller, S. J. Astrocyte heterogeneity in the adult central nervous system. Front. Cell. Neurosci. 12:1–6, 2018.
Molnár, J., et al. Transmigration characteristics of breast cancer and melanoma cells through the brain endothelium: Role of Rac and PI3K. Cell Adhes. Migr. 10:269–281, 2016.
Motallebnejad, P., and S. M. Azarin. Chemically defined human vascular laminins for biologically relevant culture of hiPSC-derived brain microvascular endothelial cells. Fluids Barriers CNS BioMed Central. 17:1–16, 2020.
Motallebnejad, P., A. Thomas, S. L. Swisher, and S. M. Azarin. An isogenic hiPSC-derived BBB-on-a-chip. Biomicrofluidics. 13:1–13, 2019.
Nibbs, R. J. B., and G. J. Graham. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 13:815–829, 2013.
Nieder, C., O. Spanne, M. P. Mehta, A. L. Grosu, and H. Geinitz. Presentation, patterns of care, and survival in patients with brain metastases: What has changed in the last 20 years? Cancer. 117:2505–2512, 2011.
O’Carroll, S. J., et al. Pro-inflammatory TNFα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J. Neuroinflammation. 12:1–18, 2015.
Pan, W., K. P. Stone, H. Hsuchou, V. K. Manda, Y. Zhang, and A. J. Kastin. Cytokine signaling modulates BBB function. Curr Pharm Des. 17:3729–3740, 2014.
Platta, C. S., D. Khuntia, M. P. Mehta, and J. H. Suh. Current treatment strategies for brain metastasis and complications from therapeutic techniques NCF in brain metastasis. Am. J. Clin. Oncol. 33:398–407, 2010.
Pranda, M. A., K. M. Gray, A. J. L. DeCastro, G. M. Dawson, J. W. Jung, and K. M. Stroka. Tumor cell mechanosensing during incorporation into the brain microvascular endothelium. Cell. Mol. Bioeng. 12:455–480, 2019.
Rajaram, M., J. Li, M. Egeblad, and R. S. Powers. System-wide analysis reveals a complex network of tumor-fibroblast interactions involved in tumorigenicity. PLoS Genet. 9:e1003789, 2013.
Rempe, R. G., A. M. S. Hartz, and B. Bauer. Matrix metalloproteinases in the brain and blood–brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 36:1481–1507, 2016.
Rieder, F., et al. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am. J. Pathol. 179:2660–2673, 2011.
Romero-Moreno, R., et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat. Commun. 10:4404, 2019.
Rostami, R., S. Mittal, P. Rostami, F. Tavassoli, and B. Jabbari. Brain metastasis in breast cancer: a comprehensive literature review. J. Neurooncol. 127:407–414, 2016.
Sadowska, G. B., et al. Interleukin-1β transfer across the blood–brain barrier in the ovine fetus. J. Cereb. Blood Flow Metab. 35:1388–1395, 2015.
Shaftel, S. S., W. S. T. Griffin, and K. M. Kerry. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J. Neuroinflammation. 5:1–12, 2008.
Shumakovich, M. A., C. P. Mencio, J. S. Siglin, R. A. Moriarty, H. M. Geller, and K. M. Stroka. Astrocytes from the brain microenvironment alter migration and morphology of metastatic breast cancer cells. FASEB J. 31:5049–5067, 2017.
Siegel, R. L., K. D. Miller, and A. Jemal. Cancer statistics, 2020. CA Cancer J. Clin. 70:7–30, 2020.
Skinner, R. A., R. M. Gibson, N. J. Rothwell, E. Pinteaux, and J. I. Penny. Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br. J. Pharmacol. 156:1115–1123, 2009.
Spampinato, S. F., V. Bortolotto, P. L. Canonico, M. A. Sortino, and M. Grilli. Astrocyte-derived paracrine signals: relevance for neurogenic niche regulation and blood–brain barrier integrity. Front. Pharmacol. 10:1–9, 2019.
Stamatovic, S. M., A. M. Johnson, R. F. Keep, and A. V. Andjelkovic. Junctional proteins of the blood–brain barrier: new insights into function and dysfunction. Tissue Barriers. 4:1–12, 2016.
Stebbins, M. J., H. K. Wilson, S. G. Canfield, T. Qian, S. P. Palecek, and E. V. Shusta. Differentiation and characterization of human pluripotent stem cell-derived brain microvascular endothelial cells. Methods. 101:93–102, 2015.
Tulotta, C., et al. Endogenous production of IL1B by breast cancer cells drives metastasis and colonization of the bone microenvironment. Clin. Cancer Res. 25:2769–2782, 2019.
Tulotta, C., and P. Ottewell. The role of IL-1B in breast cancer bone metastasis. Endocr. Relat. Cancer. 25:R421–R434, 2018.
Uhlén, M., et al. The human secretome. Sci. Signal. 12:1–9, 2019.
Vacchini, A., M. Locati, and E. M. Borroni. Overview and potential unifying themes of the atypical chemokine receptor family. J. Leukoc. Biol. 99:883–892, 2016.
Valiente, M., et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 156:1002–1016, 2014.
Wang, L., et al. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PLoS ONE. 8:e80933, 2013.
Wasilewski, D., Priego, N., Fustero-Torre, C., and M. Valiente. Reactive astrocytes in brain metastasis. Front. Oncol. 7:1–12, 2017.
Wrobel, J. K., and M. Toborek. Blood–brain barrier remodeling during brain metastasis formation. Mol. Med. 22:32–40, 2016.
Xing, F., et al. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol. Med. 5(3):384–396, 2013.
Xing, F., et al. MiR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene. 34:4890–4900, 2015.
Xing, F., et al. Activation of the c-Met pathway mobilizes an inflammatory network in the brain microenvironment to promote brain metastasis of breast cancer. Cancer Res. 76:4970–4980, 2016.
Xu, H., Z. Li, Y. Yu, S. Sizdahkhani, W. S. Ho, and F. Yin. A dynamic in vivo-like organotypic blood–brain barrier model to probe metastatic brain tumors. Sci. Rep. 6:1–12, 2016.
Yang, C., et al. CXCL1 stimulates migration and invasion in ER-negative breast cancer cells via activation of the ERK/MMP2/9 signaling axis. Int. J. Oncol. 55:684–696, 2019.
Acknowledgments
Portions of this work were conducted in the Minnesota Nano Center, which is supported by the National Science Foundation through the National Nano Coordinated Infrastructure Network (NNCI) under Award Number ECCS-2025124. Confocal microscopy and image analysis was performed using the Nikon A1Rsi Confocal microscope and NIS-Elements software at the University Imaging Center, University of Minnesota.
Conflict of interest
All authors (PM, VVR, and SMA) declare that they have no conflict of interests.
Ethical Approval
No human or animal studies were carried out by the authors for this article.
Funding
This work was supported by the University of Minnesota.
Author information
Authors and Affiliations
Contributions
PM and SMA designed the experiments. PM and VVR performed the experiments and analyzed the data. PM and SMA wrote and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Motallebnejad, P., Rajesh, V.V. & Azarin, S.M. Evaluating the Role of IL-1β in Transmigration of Triple Negative Breast Cancer Cells Across the Brain Endothelium. Cel. Mol. Bioeng. 15, 99–114 (2022). https://doi.org/10.1007/s12195-021-00710-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-021-00710-y