Abstract
Purpose
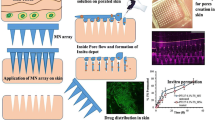
Objective of present work was to develop microfluidic-based technique for continuous production of drug (luliconazole)-loaded microemulsion.
Methods
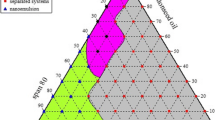
Pseudo-ternary phase diagrams were constructed, and optimization of concentration of selected formulation components like Capmul MCM, Tween 20 and Transcutol P was done using the Box-Behnken design (BBD). Batch process and microfluidic process were compared in terms of droplet size. Further, microemulsion was loaded into chitosan-based gel, and ex vivo skin permeation study was carried in comparison with marketed formulation.
Results
Optimized batch (ME 1) with Capmul MCM (10% v/v) and mixture of Tween 20 and Transcutol P (32.5% v/v) processed with flow rate of 20 mL/min through microfluidic device exhibited droplet size of 24.9 ± 3 nm (PDI 0.4 ± 0.03). Developed formulation enhanced flux of luliconazole across skin and was stable for 3 months. Increase in volume (20 to 100 mL) for batch process led to corresponding increment in droplet size (from 31.1 ± 11.9 to 56.4 nm) indicating lack of uniformity. Alternatively, for microfluidic device, such alterations in droplet size were avoided.
Conclusion
Achievement of prerequisite droplet size is imperative to colloidal stability of microemulsions and is problematic at larger batch sizes. Batch processes employed are unable to address major issue of uniformity in droplet size and ease of scale up. Experimental findings conclude that as opposed to bath processes, consistent production of microemulsion would be achieved even with higher batch size using microfluidic device. Results strongly advocate further exploration of microfluidic platforms for producing drug-loaded microemulsions.

Graphical Abstract






Similar content being viewed by others
Abbreviations
- DOE:
-
Design of Experiments
- EMG:
-
Emulgel
- BBD:
-
Box-Behnken design
- MIC:
-
Minimum inhibitory concentration
References
Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Critical Reviews™ in Therapeutic Drug Carrier Systems. 1999;16(5).
Lu GW, Gao P. Emulsions and microemulsions for topical and transdermal drug delivery. Handbook of non-invasive drug delivery systems: Elsevier; 2010. p. 59–94.
Chatzidaki MD, Mitsou E, Yaghmur A, Xenakis A, Papadimitriou V. Formulation and characterization of food-grade microemulsions as carriers of natural phenolic antioxidants. Colloids Surf A Physicochem Eng Asp. 2015;483:130–6.
Kale SN, Deore SL. Emulsion micro emulsion and nano emulsion: a review. Sys Rev Pharm. 2017;8(1):39.
Azeem A, Rizwan M, Ahmad FJ, Khan ZI, Khar RK, Aqil M, et al. Emerging role of microemulsions in cosmetics. Recent Pat Drug Deliv Formul. 2008;2(3):275–89.
Gadhave AD, Waghmare JT. A short review on microemulsion and its application in extraction of vegetable oil. Int J Res Eng Tech. 2014;3(9):147–58.
Singh V, Bushettii S, Raju AS, Ahmad R, Singh M, Bisht A. Microemulsions as promising delivery systems: a review. Ind J Pharm Edu Res. 2011;4:54.
Choi C-H, C-h C. Aqueous synthesis of tailored ZnO nanocrystals, nanocrystal assemblies, and nanostructured films by physical means enabled by a continuous flow microreactor. Cryst Growth Des. 2014;14(9):4759–67.
Zeng C, Wang C, Wang F, Zhang Y, Zhang L. A novel vapor–liquid segmented flow based on solvent partial vaporization in microstructured reactor for continuous synthesis of nickel nanoparticles. Chem Eng J. 2012;204:48–53.
Zhao C-X, He L, Qiao SZ, Middelberg AP. Nanoparticle synthesis in microreactors. Chem Eng Sci. 2011;66(7):1463–79.
Hao J, Fang X, Zhou Y, Wang J, Guo F, Li F, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Int J Nanomedicine. 2011;6:683.
Ferreira SC, Bruns R, Ferreira H, Matos G, David J, Brandao G, et al. Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597(2):179–86.
Tripathi P, Srivastava VC, Kumar A. Optimization of an azo dye batch adsorption parameters using box–Behnken design. Desalination. 2009;249(3):1273–9.
Kumar A, Prasad B, Mishra I. Process parametric study for ethene carboxylic acid removal onto powder activated carbon using Box-Behnken design. Chem Eng Technol. 2007;30(7):932–7.
Scher RK, Nakamura N, Tavakkol A. Luliconazole: a review of a new antifungal agent for the topical treatment of onychomycosis. Mycoses. 2014;57(7):389–93.
Koga H, Nanjoh Y, Makimura K, Tsuboi R. In vitro antifungal activities of luliconazole, a new topical imidazole. Med Mycol. 2009;47(6):640–7.
http://www.gbiresearch.com. Global dermatology market to 2022 – innovative pipeline and increasing uptake of biologics to diversify treatment options and drive strong growth: GBI research; [cited 2019 10/10/2019]. Available from: http://www.gbiresearch.com/report-store/market-reports/therapy-analysis/global-dermatology-market-to-2022-innovative-pipeline-and-increasing-uptake-of-biologics-to-diversify-treatment-options-and-d. Access date: 10th October 2019.
Al Abood RM, Talegaonkar S, Tariq M, Ahmad FJ. Microemulsion as a tool for the transdermal delivery of ondansetron for the treatment of chemotherapy induced nausea and vomiting. Colloids Surf B: Biointerfaces. 2013;101:143–51.
Barakat NS. Evaluation of glycofurol-based gel as a new vehicle for topical application of naproxen. AAPS PharmSciTech. 2010;11(3):1138–46.
Pawar J, Narkhede R, Amin P, Tawde V. Design and evaluation of topical diclofenac sodium gel using hot melt extrusion technology as a continuous manufacturing process with Kolliphor® P407. AAPS PharmSciTech. 2017;18(6):2303–15.
Dong C-H, Xie X-Q, Wang X-L, Zhan Y, Yao Y-J. Application of Box-Behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioprod Process. 2009;87(2):139–44.
Patil S, Krishnan RA, Bhangde S, Dandekar P, Jain R. Comparison between solid and liquid acids for production of low molecular weight chitosan using systematic DOE-based approach. Cellulose. 2018;25(10):5643–58.
Qiu P, Cui M, Kang K, Park B, Son Y, Khim E, et al. Application of Box-Behnken design with response surface methodology for modeling and optimizing ultrasonic oxidation of arsenite with H2O2. Open Chem. 2014;12(2):164–72.
Zhong K, Wang Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr Polym. 2010;80(1):19–25.
Halbaut L, Barbé C, Del Pozo A. An investigation into physical and chemical properties of semi-solid self-emulsifying systems for hard gelatin capsules. Int J Pharm. 1996;130(2):203–12.
Nazzal S, Khan MA. Response surface methodology for the optimization of ubiquinone self-nanoemulsified drug delivery system. AAPS PharmSciTech. 2002;3(1):23–31.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121.
Sugiura S, Nakajima M, Kumazawa N, Iwamoto S, Seki M. Characterization of spontaneous transformation-based droplet formation during microchannel emulsification. J Phys Chem B. 2002;106(36):9405–9.
Lippacher A, Müller R, Mäder K. Liquid and semisolid SLN™ dispersions for topical application: rheological characterization. Eur J Pharm Biopharm. 2004;58(3):561–7.
Krishnaiah YS, Xu X, Rahman Z, Yang Y, Katragadda U, Lionberger R, et al. Development of performance matrix for generic product equivalence of acyclovir topical creams. Int J Pharm. 2014;475(1–2):110–22.
EL-Hafian EA, Elgannoudi ES, Mainal A, Yahaya AHB. Characterization of chitosan in acetic acid: rheological and thermal studies. Turk J Chem. 2010;34(1):47–56.
Giles Jr HF, Mount III EM, Wagner Jr JR. Extrusion: the definitive processing guide and handbook: William Andrew; 2004.
Butani D, Yewale C, Misra A. Amphotericin B topical microemulsion: formulation, characterization and evaluation. Colloids Surf B: Biointerfaces. 2014;116:351–8.
Patel HK, Barot BS, Parejiya PB, Shelat PK, Shukla A. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo: ex vivo permeation and skin irritation studies. Colloids Surf B: Biointerfaces. 2013;102:86–94.
Sahoo S, Pani NR, Sahoo SK. Effect of microemulsion in topical sertaconazole hydrogel: in vitro and in vivo study. Drug Deliv. 2016;23(1):338–45.
Bandivadeka MM, Pancholi SS, Kaul-Ghanekar R, Choudhari A, Koppikar S. Self-microemulsifying smaller molecular volume oil (Capmul MCM) using non-ionic surfactants: a delivery system for poorly water-soluble drug. Drug Dev Ind Pharm. 2012;38(7):883–92.
Shakeel F, Baboota S, Ahuja A, Ali J, Aqil M, Shafiq S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech. 2007;8(4):191.
Binks B, Meunier J, Langevin D. Characteristic sizes, film rigidity and interfacial tensions in microemulsion systems. Progr Colloid Polym Sci: Springer; 1989. p. 208–13.
Fouad SA, Basalious EB, El-Nabarawi MA, Tayel SA. Microemulsion and poloxamer microemulsion-based gel for sustained transdermal delivery of diclofenac epolamine using in-skin drug depot: in vitro/in vivo evaluation. Int J Pharm. 2013;453(2):569–78.
Acknowledgements
The authors are also very thankful to Mr. Sanjay Solanki Virdev Intermediates Pvt. Ltd. for providing luliconazole and Mr. Shrikant Dhage (ICT Mumbai) for technical support. Special thanks to Ms. Tejal Pant for the assistance in drafting the manuscript.
Funding
The authors are thankful to the University Grants Commission for the UGC-SAP fellowship (F.25–1/2014–15 [BSR]/No. F.8–10/2007[BSR]), (SR/NM/NS-1145/2012-ICT) and DAE-ICT for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Patil, S., Pandit, A., Gaikwad, G. et al. Exploring Microfluidic Platform Technique for Continuous Production of Pharmaceutical Microemulsions. J Pharm Innov 16, 441–453 (2021). https://doi.org/10.1007/s12247-020-09457-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09457-x