Abstract
Purpose
Polyphosphazenes are synthetic polymers that contain phosphate-nitrogen molecules. Polyphosphazene’s inorganic main structure is capable of hydrolytic degradation and modulated by choosing the relevant side group. Poly[di(carboxylatophenoxy)-phosphazene (PCPP) is a polymer that is preferred due to its macromolecular structure, excellent film-forming, and microcapsule forming ability. It has biological activity when used with antigenic proteins of bacteria and viruses as microcapsule forms. Sodium chloride is added to the solution of the PCPP polymer to create coacervate microdroplets. These microdroplets are stabilized by cross-linking by adding calcium ions. PCPP is known as a biocompatible polymer; however, there are no studies on the cytotoxicity of the microparticle or water-soluble form of the PCPP.
Methods
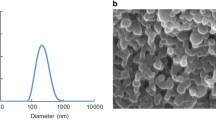
To show the compatibility of PCPP on cell culture, different concentrations of PCPP’s cytotoxic effect were investigated. The microparticle form of PCPP polymer was obtained by the coacervation method. The size of these microcapsules was shown by the dynamic light scattering. Also, the cytotoxicity of PCPP microparticles and PCPP polymer in different concentrations were examined using mouse fibroblast cell lines by XTT assay. The number of cells undergoing apoptosis was determined by the DAPI staining assay. Concentration-dependent cytotoxicity was shown by this study.
Results
PCPP microparticles and soluble form of this polymer have shown no toxic effects in studied concentrations. The average size range of the PCPP microparticles was found to be 2828 µm. This study also showed that, when ISO standards are referenced, both soluble and microparticle form of the PCPPdid not demonstrate any toxic effect on the L929 cell line.
Conclusions
PCPP is not toxic at the concentrations studied in in vitro cell culture and supports the literature regarding its biocompatibility.




Similar content being viewed by others
References
Andrianov AK, DeCollibus DP, Gillis HA, Henry HK, Marin A, Prausnitz MR, Mutwiri G. Poly [di (carboxylatophenoxy) phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci. 2009;106(45):18936–41.
Andrianov AK (Ed.). Polyphosphazenes for biomedical applications. John Wiley & Sons. 2009.
Sawant S, Shegokar R. Bone scaffolds: what’s new in nanoparticle drug delivery research?. In Nanobiomaterials in Hard Tissue Engineering (pp. 155–187). William Andrew Publishing. 2016.
Ghosh SK. Functional coatings and microencapsulation: a general perspective. Functional coatings. 2006;1–28.
Shim DH, Ko HJ, Volker G, Potter AA, Mutwiri G, Babiuk LA, Kweon MN. Efficacy of poly [di (sodium carboxylatophenoxy) phosphazene](PCPP) as mucosal adjuvant to induce protective immunity against respiratory pathogens. Vaccine. 2010;28(11):2311–7.
Andrianov AK, Marin A, Chen J. Synthesis, properties, and biological activity of poly [di (sodium carboxylatoethylphenoxy) phosphazene]. Biomacromol. 2006;7(1):394–9.
Andrianov AK, Chen J. Polyphosphazene microspheres: preparation by ionic complexation of phosphazene polyacids with spermine. J Appl Polym Sci. 2006;101(1):414–9.
Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12(11):978–90.
Baillargeon AL, Mequanint K. Biodegradable polyphosphazene biomaterials for tissue engineering and delivery of therapeutics. BioMed research intern. 2014.
Laurencin CT, Morris CD, Pierres-Jacques H, Schwartz ER, Keaton AR, Zou L. The development of bone bioerodible polymer composites for skeletal tissue regeneration: studies of initial cell attachment and spread. Polym Adv Technol. 1992;3:369–364.
Laurencin CT, Norman ME, Elgendy HM, El-Amin SF, Allcock HR, Pucher SR, Ambrosio AA. Use of polyphosphazenes for skeletal tissue regeneration. J Biomed Mater Res. 1993;27(7):963–73.
Gümüşderelioǧlu M, Gür A. Synthesis, characterization, in vitro degradation and cytotoxicity of poly [bis (ethyl 4-aminobutyro) phosphazene]. React Funct Polym. 2002;52(2):71–80.
Andrianov AK, Chen J, Payne LG. Preparation of hydrogel microspheres by coacervation of aqueous polyphosphazene solutions. Biomaterials. 1998;19(1–3):109–15.
Andrianov AK, Svirkin YY, LeGolvan MP. Synthesis and biologically relevant properties of polyphosphazene polyacids. Biomacromol. 2004;5(5):1999–2006.
Garlapati S, Eng NF, Wilson HL, Buchanan R, Mutwiri GK, Babiuk LA, Gerdts V. PCPP (poly [di (carboxylatophenoxy)-phosphazene]) microparticles co-encapsulating ovalbumin and CpG oligo-deoxynucleotides are potent enhancers of antigen specific Th1 immune responses in mice. Vaccine. 2010;28(52):8306–14.
Braakhuis HM, Park MV, Gosens I, De Jong WH, Cassee FR. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part Fibre Toxicol. 2014;11(1):18.
Cakir-Koc R, Budama-Kilinc Y, Kokcu Y, Kecel-Gunduz S. Molecular docking of immunogenic peptide of Toxoplasma gondii and encapsulation with polymer as vaccine candidate. Artificial cells, nanomedicine, and biotechnology. 2018;46(sup2):744–54.
Pajaniradje S, Mohankumar K, Pamidimukkala R, Subramanian S, Rajagopalan R. Antiproliferative and apoptotic effects of Sesbania grandiflora leaves in human cancer cells. BioMed research intern. 2014:2014.
I. S. O. (ISO), Iso 10993–5: 2009. biological evaluation of medical devices–part 5: tests for in vitro cytotoxicity, 2009.
Porter KG, Feig YS. The use of DAPI for identifying and counting aquatic microflora 1. Limnol Oceanogr. 1980;25(5):943–8.
Temel M. Bazı diamin ve dihidroksi türevli, ağ yapılı polifosfazen mikrokürelerin sentezi ve yapısal özelliklerinin incelenmesi (Master's thesis, Bilecik Şeyh Edebali Üniversitesi, Fen Bilimleri Enstitüsü). 2015.
Rothemund S, Teasdale I. Preparation of polyphosphazenes: a tutorial review. Chem Soc Rev. 2016;45(19):5200–15.
Funding
This work was supported by the TUBITAK- 3001—Startup Research and Development Projects Support Program (Grant Number: 118S721).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ustun-Karatop, E., Cakır-Koc, R. Biocompatibility of Poly[di(carboxylatophenoxy)-phosphazene] Polymer: In Vitro Cytotoxicity in Cell Culture. J Pharm Innov 17, 1199–1204 (2022). https://doi.org/10.1007/s12247-021-09598-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09598-7