Abstract
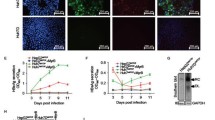
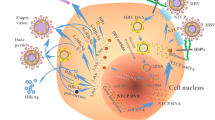
The construction of the first infectious clone JFH-1 speeds up the research on hepatitis C virus (HCV). However, Huh7 cell line was the only highly permissive cell line for HCV infection and only a few clones were fully permissive. In this study, two different fully permissive clones of Huh7 cells, Huh7.5.1 and Huh7-Lunet-CD81 (Lunet-CD81) cells were compared for their responses upon HCV infection. The virus replication level was found slightly higher in Huh7.5.1 cells than that in Lunet-CD81 cells. Viability of Huh7.5.1 cells but not of Lunet-CD81 cells was reduced significantly after HCV infection. Further analysis showed that the cell cycle of infected Huh7.5.1 cells was arrested at G1 phase. The G1/S transition was blocked by HCV infection in Huh7.5.1 cells as shown by the cell cycle synchronization analysis. Genes related to cell cycle regulation was modified by HCV infection and gene interaction analysis in GeneSpring GX in Direct Interactions mode highlighted 31 genes. In conclusion, the responses of those two cell lines were different upon HCV infection. HCV infection blocked G1/S transition and cell cycle progress, thus reduced the cell viability in Huh7.5.1 cells but not in Lunet-CD81 cells. Lunet-CD81 cells might be suitable for long term infection studies of HCV.
Similar content being viewed by others
References
Alisi A, Mele R, Spaziani A, Tavolaro S, Palescandolo E, and Balsano C. 2005. Thr 446 phosphorylation of PKR by HCV core protein deregulates G2/M phase in HCC cells. J Cell Physiol, 205: 25–31.
Bartenschlager R, and Pietschmann T. 2005. Efficient hepatitis C virus cell culture system: what a difference the host cell makes. Proc Natl Acad Sci U S A, 102: 9739–9740.
Blight K J, McKeating J A, and Rice C M. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol, 76: 13001–13014.
Brault C, Levy P L, and Bartosch B. 2013. Hepatitis C virus-induced mitochondrial dysfunctions. Viruses, 5: 954–980.
Deng L, Adachi T, Kitayama K, Bungyoku Y, Kitazawa S, Ishido S, Shoji I, and Hotta H. 2008. Hepatitis C virus infection induces apoptosis through a Bax-triggered, mitochondrion-mediated, caspase 3-dependent pathway. J Virol, 82: 10375–10385.
Friebe P, Boudet J, Simorre J-P, and Bartenschlager R. 2005. Kissing-Loop Interaction in the 3′ End of the Hepatitis C Virus Genome Essential for RNA Replication. J Virol, 79: 380–392.
Han Q, Xu C, Wu C, Zhu W, Yang R, and Chen X. 2009. Compensatory mutations in NS3 and NS5A proteins enhance the virus production capability of hepatitis C reporter virus. Virus Res, 145: 63–73.
Joyce M A, Walters K A, Lamb S E, Yeh M M, Zhu L F, Kneteman N, Doyle J S, Katze M G, and Tyrrell D L. 2009. HCV induces oxidative and ER stress, and sensitizes infected cells to apoptosis in SCID/Alb-uPA mice. PLoS Pathog, 5: e1000291.
Kannan R P, Hensley L L, Evers L E, Lemon S M, and McGivern D R. 2011. Hepatitis C virus infection causes cell cycle arrest at the level of initiation of mitosis. J Virol, 85: 7989–8001.
Ke P Y, and Chen S S. 2012. Hepatitis C virus and cellular stress response: implications to molecular pathogenesis of liver diseases. Viruses, 4: 2251–2290.
Koutsoudakis G, Herrmann E, Kallis S, Bartenschlager R, and Pietschmann T. 2007. The level of CD81 cell surface expression is a key determinant for productive entry of hepatitis C virus into host cells. J Virol, 81: 588–598.
Li K, Foy E, Ferreon J C, Nakamura M, Ferreon A C, Ikeda M, Ray S C, Gale M, Jr., and Lemon S M. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A, 102: 2992–2997.
Lindenbach B D, Evans M J, Syder A J, Wolk B, Tellinghuisen T L, Liu C C, Maruyama T, Hynes R O, Burton D R, McKeating J A, and Rice C M. 2005. Complete replication of hepatitis C virus in cell culture. Science, 309: 623–626.
Liu J, Ding X, Tang J, Cao Y, Hu P, Zhou F, Shan X, Cai X, Chen Q, Ling N, Zhang B, Bi Y, Chen K, Ren H, Huang A, He T C, and Tang N. 2011. Enhancement of canonical Wnt/beta-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma cells. PLoS One, 6: e27496.
Manns M P, McHutchison J G, Gordon S C, Rustgi V K, Shiffman M, Reindollar R, Goodman Z D, Koury K, Ling M, and Albrecht J K. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet, 358: 958–965.
Murayama A, Sugiyama N, Yoshimura S, Ishihara-Sugano M, Masaki T, Kim S, Wakita T, Mishiro S, and Kato T. 2012. A subclone of HuH-7 with enhanced intracellular hepatitis C virus production and evasion of virus related-cell cycle arrest. PLoS One, 7: e52697.
Pei R, Chen H, Lu L, Zhu W, Beckebaum S, Cicinnati V, Lu M, and Chen X. 2011. Hepatitis C virus infection induces the expression of amphiregulin, a factor related to the activation of cellular survival pathways and required for efficient viral assembly. J Gen Virol, 92: 2237–2248.
Pokrovskii M V, Bush C O, Beran R K F, Robinson M F, Cheng G, Tirunagari N, Fenaux M, Greenstein A E, Zhong W, Delaney W E, and Paulson M S. 2011. Novel Mutations in a Tissue Culture-Adapted Hepatitis C Virus Strain Improve Infectious-Virus Stability and Markedly Enhance Infection Kinetics. J Virol, 85: 3978–3985.
Rakela J, and Vargas H E. 2002. Hepatitis C: magnitude of the problem. Liver Transpl, 8: S3–6.
Sainz B, Jr., Barretto N, and Uprichard S L. 2009. Hepatitis C virus infection in phenotypically distinct Huh7 cell lines. PLoS One, 4: e6561.
Sumpter R, Loo Y-M, Foy E, Li K, Yoneyama M, Fujita T, Lemon S M, and Gale M. 2005. Regulating Intracellular Antiviral Defense and Permissiveness to Hepatitis C Virus RNA Replication through a Cellular RNA Helicase, RIG-I. J Virol, 79: 2689–2699.
Walters K-A, Syder A J, Lederer S L, Diamond D L, Paeper B, Rice C M, and Katze M G. 2009. Genomic Analysis Reveals a Potential Role for Cell Cycle Perturbation in HCV-Mediated Apoptosis of Cultured Hepatocytes. PLoS Pathog, 5: e1000269.
Walters K A, Syder A J, Lederer S L, Diamond D L, Paeper B, Rice C M, and Katze M G. 2009. Genomic analysis reveals a potential role for cell cycle perturbation in HCV-mediated apoptosis of cultured hepatocytes. PLoS Pathog, 5: e1000269.
Wang Q, Chen J, Wang Y, Han X, and Chen X. 2012. Hepatitis C virus induced a novel apoptosis-like death of pancreatic beta cells through a caspase 3-dependent pathway. PLoS One, 7: e38522.
Wang Y, Xu Y, Tong W, Pan T, Li J, Sun S, Shao J, Ding H, Toyoda T, and Yuan Z. 2011. Hepatitis C virus NS5B protein delays s phase progression in human hepatocyte-derived cells by relocalizing cyclin-dependent kinase 2-interacting protein (CINP). J Biol Chem, 286: 26603–26615.
Yang X J, Liu J, Ye L, Liao Q J, Wu J G, Gao J R, She Y L, Wu Z H, and Ye L B. 2006. HCV NS2 protein inhibits cell proliferation and induces cell cycle arrest in the S-phase in mammalian cells through down-regulation of cyclin A expression. Virus Res, 121: 134–143.
Zhong J, Gastaminza P, Chung J, Stamataki Z, Isogawa M, Cheng G, McKeating J A, and Chisari F V. 2006. Persistent hepatitis C virus infection in vitro: coevolution of virus and host. J Virol, 80: 11082–11093.
Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton D R, Wieland S F, Uprichard S L, Wakita T, and Chisari F V. 2005. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A, 102: 9294–9299.
Zhu H, Dong H, Eksioglu E, Hemming A, Cao M, Crawford J M, Nelson D R, and Liu C. 2007. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology, 133: 1649–1659.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Pei, R. & Chen, X. Different responses of two highly permissive cell lines upon HCV infection. Virol. Sin. 28, 202–208 (2013). https://doi.org/10.1007/s12250-013-3342-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-013-3342-5