Summary
Background
Febrile neutropenia (FN) is a common side effect of chemotherapy that frequently necessitates hospitalization and healthcare resource utilization (HCRU), but is poorly studied in Eastern European countries. We investigated HCRU and granulocyte colony-stimulating factor (G-CSF) use in patients hospitalized for FN in Bulgaria, Czech Republic, and Slovakia.
Patients and methods
This was a multicenter retrospective cohort study. Eligible patients were ≥18 years old, had received chemotherapy for solid tumors or hematological malignancies of any stage, and had been hospitalized for FN. The primary objective was to evaluate FN-related HCRU; secondary objectives included the description of chemotherapy treatment patterns and G‑CSF use. Data were analyzed by participating country.
Results
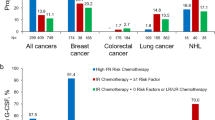
Data of 156 patients from Bulgaria and 79 patients each from the Czech Republic and Slovakia were analyzed. The most frequent solid tumors were breast (n = 28) and testicular cancer (n = 13), and the most common hematological malignancies were non-Hodgkin B‑cell lymphoma (n = 51) and acute myeloid leukemia (n = 35). In general, G‑CSF was used to treat FN rather than as prophylaxis. Most patients had a single FN episode, predominantly in cycle 1. The mean duration of FN-related hospitalization was 7–9 days, with longer stays in patients with hematological malignancies.
Conclusions
Results indicate considerable FN-related HCRU in all countries. Frequent lack of G‑CSF primary prophylaxis was observed, particularly in Slovakia.
Similar content being viewed by others
Abbreviations
- ANC:
-
absolute neutrophil count
- CEE:
-
Central Eastern Europe
- EORTC:
-
European Organization for Research and Treatment of Cancer
- FN:
-
febrile neutropenia
- G-CSF:
-
granulocyte colony-stimulating factor
- HCRU:
-
healthcare resource utilization
- NHL:
-
non-Hodgkin’s lymphoma
References
Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228–37.
Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14(1):29–35.
Holmes FA, Jones SE, O’Shaughnessy J, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13(6):903–9.
Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20(3):727–31.
Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32.
Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–66.
Hirsch BR, Lyman GH. Pharmacoeconomics of the myeloid growth factors: a critical and systematic review. Pharmacoeconomics. 2012;30(6):497–511.
Schilling MB, Parks C, Deeter RG. Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: A retrospective study. Exp Ther Med. 2011;2(5):859–66.
Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21(24):4524–31.
Weycker D, Danel A, Bendall K, et al. Cost of chemotherapy-induced febrile neutropenia treated in inpatient, outpatient, and home care settings among adults with non-Hodgkin’s lymphoma in European clinical practice. Blood. 2011;118:4200, ASH Annual Meeting Abstracts.
Weycker D, Barron R, Kartashov A, et al. Incidence, treatment, and consequences of chemotherapy-induced febrile neutropenia in the inpatient and outpatient settings. J Oncol Pharm Pract. 2014;20(3):190–8.
Saramago P, Andreozzi V, Ferreira JM, et al. Modelling the direct costs of chemotherapy-induced neutropenia treatment in Portuguese hospitals clinical practice. J Clin Oncol. 2007;25(18S):17081, ASCO Annual Meeting Proceedings (Post-Meeting Edition), June 20 Supplement.
Mayordomo JI, Lopez A, Vinolas N, et al. Retrospective cost analysis of management of febrile neutropenia in cancer patients in Spain. Curr Med Res Opin. 2009;25(10):2533–42.
Ihbe-Heffinger A, Paessens BJ, von Schilling C, et al. Management of febrile neutropenia – a German prospective hospital cost analysis in lymphoproliferative disorders, non-small cell lung cancer, and primary breast cancer. Onkologie. 2011;34(5):241–6.
Moeremans K, Caekelbergh K, Spaepen E. et al. Economic aspects and drivers of febrile neutropenia in cancer – a multicentre retrospective analysis in Belgium. ISPOR 8th Annual European Congress, Florence. 2005.
Liu Z, Doan QV, Malin J, et al. The economic value of primary prophylaxis using pegfilgrastim compared with filgrastim in patients with breast cancer in the UK. Appl Health Econ Health Policy. 2009;7(3):193–205.
Vainchtock A, Cohen-Nizard S, Durand-Zaleski I. Analysis of public and private hospital databases (PMSI) 2010/2011 to estimate the frequency and associated costs for febrile neutropenia in france. ISPOR 16th Annual European Congress, Dublin. 2013.
Mattli R, Pletscher M, Eichler K. et al. Inpatient hospital costs of febrile neutropenia as a consequence of chemotherapy for breast cancer and Non-Hodgkin Lymphoma in Switzerland. ISPOR 16th Annual European Congress, Dublin. 2013.
Liou SY, Stephens JM, Carpiuc KT, et al. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig. 2007;27(6):381–96.
Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–67.
Caggiano V, Weiss RV, Rickert TS, et al. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103(9):1916–24.
Kuderer NM. Meta-analysis of randomized controlled trials of granulocyte colony-stimulating factor prophylaxis in adult cancer patients receiving chemotherapy. Cancer Treat Res. 2011;157:127–43.
Crawford J, Glaspy JA, Stoller RG, et al. Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: exploration of risk factors for febrile neutropenia. Support Cancer Ther. 2005;3(1):36–46.
Fiegl M, Steger GG, Studnicka M, et al. Pegfilgrastim prophylaxis in patients at different levels of risk for chemotherapy-associated febrile neutropenia: an observational study. Curr Med Res Opin. 2013;29(5):505–15.
Maenpaa J, Varthalitis I, Erdkamp F, et al. The use of granulocyte colony stimulating factor (G-CSF) and management of chemotherapy delivery during adjuvant treatment for early-stage breast cancer – further observations from the IMPACT solid study. Breast. 2016;25:27–33.
World Health Organization. Access to new medicines in Europe: technical review of policy initiatives and opportunities for collaboration and research 2015. http://www.euro.who.int/__data/assets/pdf_file/0008/306179/Access-new-medicines-TR-PIO-collaboration-research.pdf?ua=1. Accessed 20 July 2015.
Acknowledgements
The authors would like to thank the study investigators for participating and enrolling patients into the study. Medical writing assistance was provided by Margit Hemetsberger, hemetsberger medical services, Vienna, Austria, and Olga Garbuzenko, Quartesian, Princeton, NJ, USA.
Funding
This study was funded by Amgen Central Eastern Europe Headoffice, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Mihaylov, Z. Mihaylova, A. Cipkova, and J. Novak declare that they have no competing interests. R. Petrova is an employee of Amgen s.r.o., Sofia, Bulgaria. L. Drgona declares to have received payments from Amgen for patient recruitment and for consultation during study preparation, honoraria for presentations from Sandoz, and consultation fees and honoraria for presentations from Teva.
Additional information
Prior conference presentations: Mihaylov G, Mihaylova Z, Drgona L, Cipkova A, Novak J, Petrova R. Healthcare resource utilization (HCRU) in hospitalized febrile neutropenia (FN) patients treated with chemotherapy for solid tumors (ST) and hematological malignancies (HM) in Bulgaria (BG), Czech Republic (CZ) and Slovakia (SK) as observed in clinical practice. ISPOR 17th Annual European Congress, Amsterdam, The Netherlands, 8–12 November 2014; abstract and poster code PCN245. Gercheva L, Goranov S, Raynov J, Mihaylova Z, Mihaylov G, Karanikolov S, Petrova R. Healthcare resource utilization (HCRU) in hospitalized febrile neutropenia (FN) patients treated with chemotherapy for solid tumors (ST) and hematological malignancies (HM) in Bulgaria. ISPOR 17th Annual European Congress, Amsterdam, The Netherlands, 8–12 November 2014; abstract and poster code PCN238
Caption Electronic Supplementary Material
12254_2016_279_MOESM1_ESM.docx
The Electronic Supplementary Material is intended to support payors, decision makers, and health economics experts to make their own cost calculations. It contains details on the specific healthcare resource use variables on which data was collected. It also contains detailed tables of the findings and sensitivity analyses.
Rights and permissions
About this article
Cite this article
Mihaylov, G., Mihaylova, Z., Drgona, L. et al. Healthcare resource utilization and G‑CSF use in patients with solid tumors or hematological malignancies hospitalized for febrile neutropenia in Bulgaria, Czech Republic and Slovakia. memo 9, 144–152 (2016). https://doi.org/10.1007/s12254-016-0279-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-016-0279-z