Abstract
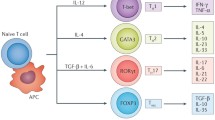
Bile acid receptor TGR5 has been proved to play protective roles in the process of myocardial infarction (MI). Recently, we found spleen weight of Tgr5+/+ mice was increased at 7-day post-MI but not in Tgr5−/− mice. Since the spleen is one of the main resources of immune and inflammatory cells post-MI, we conducted flow cytometry analysis of multiple immune cells in the heart post-MI. It showed the recruitment of CD4+ T cells and CD8+ T cells was continuously more in the heart of Tgr5−/− mice post-MI until 7 days after MI. Furthermore, CD4-specific TGR5 depletion mice exhibited aggravated ischemic injury. The mRNA expressions of the markers of Th1 and Treg were upregulated in the heart of Tgr5−/− mice at 7-day post-MI. These results suggested TGR5 modulates CD4+ T cell functions and subsets distribution in the heart, and plays protective roles in myocardial infarction.
Graphical abstract







Similar content being viewed by others
References
Bansal, S. S., Ismahil, M. A., Goel, M., Zhou, G., Rokosh, G., Hamid, T., & Prabhu, S. D. (2019). Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation, 139(2), 206–221. https://doi.org/10.1161/CIRCULATIONAHA.118.036065
Zhang, S., Bories, G., Lantz, C., Emmons, R., Becker, A., Liu, E., Abecassis, M. M., Yvan-Charvet, L., & Thorp, E. B. (2019). Immunometabolism of phagocytes and relationships to cardiac repair. Frontiers in Cardiovascular Medicine, 6, 42. https://doi.org/10.3389/fcvm.2019.00042
Weil, B. R., & Neelamegham, S. (2019). Selectins and immune cells in acute myocardial infarction and post-infarction ventricular remodeling: Pathophysiology and novel treatments. Frontiers in Immunology, 10, 300. https://doi.org/10.3389/fimmu.2019.00300
Wang, J., Zhang, J., Lin, X., Wang, Y., Wu, X., Yang, F., Gao, W., Zhang, Y., Sun, J., Jiang, C., & Xu, M. (2021). DCA-TGR5 signaling activation alleviates inflammatory response and improves cardiac function in myocardial infarction. Journal of Molecular and Cellular Cardiology, 151, 3–14. https://doi.org/10.1016/j.yjmcc.2020.10.014
Perino, A., Pols, T. W., Nomura, M., Stein, S., Pellicciari, R., & Schoonjans, K. (2014). TGR5 reduces macrophage migration through mTOR-induced C/EBPbeta differential translation. The Journal of Clinical Investigation, 124(12), 5424–5436. https://doi.org/10.1172/JCI76289
Hogenauer, K., Arista, L., Schmiedeberg, N., Werner, G., Jaksche, H., Bouhelal, R., Nguyen, D. G., Bhat, B. G., Raad, L., Rauld, C., & Carballido, J. M. (2014). G-protein-coupled bile acid receptor 1 (GPBAR1, TGR5) agonists reduce the production of proinflammatory cytokines and stabilize the alternative macrophage phenotype. Journal of Medicinal Chemistry, 57(24), 10343–10354. https://doi.org/10.1021/jm501052c
Biagioli, M., Carino, A., Cipriani, S., Francisci, D., Marchiano, S., Scarpelli, P., Sorcini, D., Zampella, A., & Fiorucci, S. (2017). The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis. The Journal of Immunology, 199(2), 718–733. https://doi.org/10.4049/jimmunol.1700183
Pols, T. W., Nomura, M., Harach, T., Lo Sasso, G., Oosterveer, M. H., Thomas, C., Rizzo, G., Gioiello, A., Adorini, L., Pellicciari, R., Auwerx, J., & Schoonjans, K. (2011). TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metabolism, 14(6), 747–57. https://doi.org/10.1016/j.cmet.2011.11.006
Guo, C., Xie, S., Chi, Z., Zhang, J., Liu, Y., Zhang, L., Zheng, M., Zhang, X., Xia, D., Ke, Y., Lu, L., & Wang, D. (2016). Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity, 45(4), 802–816. https://doi.org/10.1016/j.immuni.2016.09.008
Gao, E., Lei, Y. H., Shang, X., Huang, Z. M., Zuo, L., Boucher, M., Fan, Q., Chuprun, J. K., Ma, X. L., & Koch, W. J. (2010). A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circulation Research, 107(12), 1445–1453. https://doi.org/10.1161/CIRCRESAHA.110.223925
Libby, P., Nahrendorf, M., & Swirski, F. K. (2016). Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: An expanded “cardiovascular continuum.” Journal of the American College of Cardiology, 67(9), 1091–1103. https://doi.org/10.1016/j.jacc.2015.12.048
Yan, X., Anzai, A., Katsumata, Y., Matsuhashi, T., Ito, K., Endo, J., Yamamoto, T., Takeshima, A., Shinmura, K., Shen, W., Fukuda, K., & Sano, M. (2013). Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. Journal of Molecular and Cellular Cardiology, 62, 24–35. https://doi.org/10.1016/j.yjmcc.2013.04.023
Bostan, M. M., Statescu, C., Anghel, L., Serban, I. L., Cojocaru, E., & Sascau, R. (2020). Post-myocardial infarction ventricular remodeling biomarkers-the key link between pathophysiology and clinic. Biomolecules, 10(11). https://doi.org/10.3390/biom10111587.
Vieira, J. M., Norman, S., Villa Del Campo, C., Cahill, T. J., Barnette, D. N., Gunadasa-Rohling, M., Johnson, L. A., Greaves, D. R., Carr, C. A., Jackson, D. G., & Riley, P. R. (2018). The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. The Journal of Clinical Investigation, 128(8), 3402–3412. https://doi.org/10.1172/JCI97192
Prabhu, S. D., & Frangogiannis, N. G. (2016). The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circulation Research, 119(1), 91–112. https://doi.org/10.1161/CIRCRESAHA.116.303577
Frangogiannis, N. G. (2014). The inflammatory response in myocardial injury, repair, and remodelling. Nature Reviews. Cardiology, 11(5), 255–265. https://doi.org/10.1038/nrcardio.2014.28
Heusch, G. (2019). The spleen in myocardial infarction. Circulation Research, 124(1), 26–28. https://doi.org/10.1161/CIRCRESAHA.118.314331
Guo, C., Chen, W. D., & Wang, Y. D. (2016). TGR5, not only a metabolic regulator. Frontiers in Physiology, 7, 646. https://doi.org/10.3389/fphys.2016.00646
Chang, S., Kim, Y. H., Kim, Y. J., Kim, Y. W., Moon, S., Lee, Y. Y., Jung, J. S., Kim, Y., Jung, H. E., Kim, T. J., Cheong, T. C., Moon, H. J., Cho, J. A., Kim, H. R., Han, D., Na, Y., Seok, S. H., Cho, N. H., Lee, H. C., … Seong, S. Y. (2018). Taurodeoxycholate increases the number of myeloid-derived suppressor cells that ameliorate sepsis in mice. Frontiers in Immunology, 9, 1984. https://doi.org/10.3389/fimmu.2018.01984
Alemi, F., Kwon, E., Poole, D. P., Lieu, T., Lyo, V., Cattaruzza, F., Cevikbas, F., Steinhoff, M., Nassini, R., Materazzi, S., Guerrero-Alba, R., Valdez-Morales, E., Cottrell, G. S., Schoonjans, K., Geppetti, P., Vanner, S. J., Bunnett, N. W., & Corvera, C. U. (2013). The TGR5 receptor mediates bile acid-induced itch and analgesia. The Journal of Clinical Investigation, 123(4), 1513–1530. https://doi.org/10.1172/JCI64551
Merlen, G., Bidault-Jourdainne, V., Kahale, N., Glenisson, M., Ursic-Bedoya, J., Doignon, I., Garcin, I., Humbert, L., Rainteau, D., & Tordjmann, T. (2020). Hepatoprotective impact of the bile acid receptor TGR5. Liver International, 40(5), 1005–1015. https://doi.org/10.1111/liv.14427
Sorrentino, G., Perino, A., Yildiz, E., El Alam, G., Sleiman, M. B., Gioiello, A., Pellicciari, R., & Schoonjans, K. (2020). Bile acids signal via TGR5 to activate intestinal stem cells and epithelial regeneration. Gastroenterology. https://doi.org/10.1053/j.gastro.2020.05.067
Lewis, N. D., Patnaude, L. A., Pelletier, J., Souza, D. J., Lukas, S. M., King, F. J., Hill, J. D., Stefanopoulos, D. E., Ryan, K., Desai, S., Skow, D., Kauschke, S. G., Broermann, A., Kuzmich, D., Harcken, C., Hickey, E. R., & Modis, L. K. (2014). A GPBAR1 (TGR5) small molecule agonist shows specific inhibitory effects on myeloid cell activation in vitro and reduces experimental autoimmune encephalitis (EAE) in vivo. PLoS One, 9(6), e100883. https://doi.org/10.1371/journal.pone.0100883
Cesta, M. F. (2006). Normal structure, function, and histology of the spleen. Toxicologic Pathology, 34(5), 455–465. https://doi.org/10.1080/01926230600867743
Methe, H., Brunner, S., Wiegand, D., Nabauer, M., Koglin, J., & Edelman, E. R. (2005). Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. Journal of the American College of Cardiology, 45(12), 1939–1945. https://doi.org/10.1016/j.jacc.2005.03.040
Tang, T. T., Zhu, Y. C., Dong, N. G., Zhang, S., Cai, J., Zhang, L. X., Han, Y., Xia, N., Nie, S. F., Zhang, M., Lv, B. J., Jiao, J., Yang, X. P., Hu, Y., Liao, Y. H., & Cheng, X. (2019). Pathologic T-cell response in ischaemic failing hearts elucidated by T-cell receptor sequencing and phenotypic characterization. European Heart Journal, 40(48), 3924–3933. https://doi.org/10.1093/eurheartj/ehz516
Bansal, S. S., Ismahil, M. A., Goel, M., Patel, B., Hamid, T., Rokosh, G., & Prabhu, S. D. (2017). Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circulation. Heart Failure, 10(3), e003688. https://doi.org/10.1161/CIRCHEARTFAILURE.116.003688
Weirather, J., Hofmann, U. D., Beyersdorf, N., Ramos, G. C., Vogel, B., Frey, A., Ertl, G., Kerkau, T., & Frantz, S. (2014). Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circulation Research, 115(1), 55–67. https://doi.org/10.1161/CIRCRESAHA.115.303895
Matsumoto, K., Ogawa, M., Suzuki, J., Hirata, Y., Nagai, R., & Isobe, M. (2011). Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. International Heart Journal, 52(6), 382–387. https://doi.org/10.1536/ihj.52.382
Hofmann, U., & Frantz, S. (2016). Role of T-cells in myocardial infarction. European Heart Journal, 37(11), 873–879. https://doi.org/10.1093/eurheartj/ehv639
Pan, W., Zhu, Y., Meng, X., Zhang, C., Yang, Y., & Bei, Y. (2019). Immunomodulation by exosomes in myocardial infarction. Journal of Cardiovascular Translational Research, 12(1), 28–36. https://doi.org/10.1007/s12265-018-9836-7
Curato, C., Slavic, S., Dong, J., Skorska, A., Altarche-Xifro, W., Miteva, K., Kaschina, E., Thiel, A., Imboden, H., Wang, J., Steckelings, U., Steinhoff, G., Unger, T., & Li, J. (2010). Identification of noncytotoxic and IL-10-producing CD8+AT2R+ T cell population in response to ischemic heart injury. The Journal of Immunology, 185(10), 6286–6293. https://doi.org/10.4049/jimmunol.0903681
Ilatovskaya, D. V., Pitts, C., Clayton, J., Domondon, M., Troncoso, M., Pippin, S., & DeLeon-Pennell, K. Y. (2019). CD8(+) T-cells negatively regulate inflammation post-myocardial infarction. American Journal of Physiology. Heart and Circulatory Physiology, 317(3), H581–H596. https://doi.org/10.1152/ajpheart.00112.2019
Varda-Bloom, N., Leor, J., Ohad, D. G., Hasin, Y., Amar, M., Fixler, R., Battler, A., Eldar, M., & Hasin, D. (2000). Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. Journal of Molecular and Cellular Cardiology, 32(12), 2141–2149. https://doi.org/10.1006/jmcc.2000.1261
Acknowledgements
We greatly appreciate Professor Changtao Jiang, School of Basic Medical Sciences, Peking University, for providing valuable opinions and suggestions on the study, especially the Tgr5 global deletion mice.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81900315, Grant No. 81625001) and the National Key Research & Development Program of China (Grant No. 2018YFC1312700, Grant No. 2018YFC1312701).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Jiaxing Wang and Tan Xu. The first draft of the manuscript was written by Jiaxing Wang and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
No human studies were carried out by the authors for this article. This study conformed to the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health and was approved by the Institutional Animal Care and Use Committee at Peking University Health Science Center (LA2019138, LA2021363).
Conflict of Interest
The authors declare no competing interest.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Xu, T. & Xu, M. Roles and Mechanisms of TGR5 in the Modulation of CD4+ T Cell Functions in Myocardial Infarction. J. of Cardiovasc. Trans. Res. 15, 350–359 (2022). https://doi.org/10.1007/s12265-021-10164-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-021-10164-2