Abstract
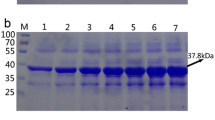
l-Arginase, hydrolyzing l-arginine to l-ornithine and urea, is a powerful anticancer, l-arginine-depleting agent, against argininosuccinate synthase expressing tumors. Otherwise, the higher antigenicity and lower thermal stability of this enzyme was the main biochemical hurdles. Since, the intrinsic thermal stability of enzymes follow the physiological temperature of their producer, thus, characterization of l-arginase from thermotolerant Penicillium chrysogenum was the objective of this study. l-Arginase (Arg) was purified to its homogeneity from P. chrysogenum by 10.1-fold, with 37.0 kDa under denaturing PAGE, optimum reaction at 50 °C, pH stability (6.8–7.9), with highest molar ratio of constitutional arginine, glutamic acid, lysine and aspartic acid. The purified enzyme was PEGylated and immobilized on chitosan, with 41.9 and 22.1 % yield of immobilization. At 40 °C, the T1/2 value of free-Arg, PEG-Arg and Chit-Arg was 10.4, 15.6, 20.5 h, respectively. The free-Arg and Chit-Arg have a higher affinity to l-arginine (K m 4.8 mM), while, PEG-Arg affinity was decreased by about 3 fold (K m 15.2 mM). The inhibitory constants to the free and PEG-Arg were relatively similar towards HA and PPG. The IC50 for the free enzyme against HEPG-2 and A549 tumor cells was 0.136 and 0.165 U/ml, comparing to 0.232 and 0.496 U/ml for PEG-Arg, respectively. The in vivo T1/2 to the free Arg and PEG-Arg was 16.4 and 20.4 h, respectively as holo-enzyme. The residual l-arginine level upon using free Arg was 156.9 and 144.5 µM, after 6 and 8 h, respectively, regarding to initials at 253.6 µM, while for Peg-Arg the level of l-arginine was nil till 7 h of initial dosing. The titer of IgG was induced by 10–15 % in response to free-Arg after 28 days comparing to IgG titer for PEG-Arg.




Similar content being viewed by others
References
Argos, P., M.G. Rossman, U.M. Grau, H. Zuber, G. Frank, and J.D. Tratschin. 1979. Thermal stability and protein structure. Biochemistry 18(25): 5698–5703.
Borkovich, K.A., and R.L. Weiss. 1987. Purification and characterization of arginase from Neurospora crassa. Journal of Biological Chemistry 262(15): 7081–7086.
Borsuk, P., A. Dzikowska, J. Empel, A. Grzelak, R. Grzeskowiak, and P. Weglenski. 1999. Structure of the arginase coding gene and its transcript in Aspergillus nidulans. Acta Biochimica Polonica 46(2): 391–403.
Boutin, J.P. 1982. Purification, properties and subunit structure of arginase from Iris bulbs. European Journal of Biochemistry 127(2): 237–243.
Cavanaugh, P.G., and G.L. Nicolson. 2000. Partial purification of a liver-derived tumor cell growth inhibitor that differentially inhibits poorly-liver metastasizing cell lines: Identification as an active subunit of arginase. Clinical & Experimental Metastasis 18(6): 509–518.
Cheng, P.N., T.L. Lam, W.M. Lam, S.M. Tsui, A.W. Cheng, W.H. Lo, and Y.C. Leung. 2007. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Research 67(1): 309–317.
Cheng, Y., and W.H. Prusoff. 1973. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochemical Pharmacology 22(23): 3099–3108.
Currie, G.A., L. Gyure, and L. Cifuentes. 1979. Microenvironmental arginine depletion by macrophages in vivo. British Journal of Cancer 39(6): 613–620.
Delage, B., D.A. Fennell, L. Nicholson, I. McNeish, N.R. Lemoine, T. Crook, and P.W. Szlosarek. 2010. Argionlinenine deprivation and argininosuccinate synthetase expression in the treatment of cancer. International Journal of Cancer 126(12): 2762–2772.
Dzikowska, A., J.P. Le Caer, P. Jonczyk, and P. Weglenski. 1994. Purification of arginase from Aspergillus nidulans. Acta Biochimica Polonica 41(4): 467–471.
Edwards, P., J.C. Cendan, D.B. Topping, L.L. Moldawer, S. MacKay, E. Copeland, and D.S. Lind. 1996. Tumor cell nitric oxide inhibits cell growth in vitro, but stimulates tumorigenesis and experimental lung metastasis in vivo. Journal of Surgical Research 63(1): 49–52.
El-Sayed, A.S. 2010. Microbial L-methioninase: Production, molecular characterization, and therapeutic applications. Applied Microbiology and Biotechnology 86(2): 445–467.
El-Sayed, A.S. 2011. Purification and characterization of a new L-methioninase from solid cultures of Aspergillus flavipes. J Microbiol 49(1): 130–140.
El-Sayed, A.S., H. Ibrahim, and M.Z. Sitohy. 2014. Co-immobilization of PEGylated Aspergillus flavipes L-methioninase with glutamate dehydrogenase: A novel catalytically stable anticancer consortium. Enyzme and Microbial Technology 54: 59–69.
El-Sayed, A.S., S.A. Khalaf, and H.A. Aziz. 2013. Characterization of homocysteine gamma-lyase from submerged and solid cultures of Aspergillus fumigatus ASH (JX006238). Journal of Microbiology and Biotechnology 23(4): 499–510.
El-Sayed, A.S., and A.A. Shindia. 2011. Characterization and immobilization of purified Aspergillus flavipesl-methioninase: Continuous production of methanethiol. Journal of Applied Microbiology 111(1): 54–69.
El-Sayed, A.S., S.A. Shouman, and H.M. Nassrat. 2012. Pharmacokinetics, immunogenicity and anticancer efficiency of Aspergillus flavipes L-methioninase. Enyzme and Microbial Technology 51(4): 200–210.
Ensor, C.M., F.W. Holtsberg, J.S. Bomalaski, and M.A. Clark. 2002. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Research 62(19): 5443–5450.
Farias-Eisner, R., M.P. Sherman, E. Aeberhard, and G. Chaudhuri. 1994. Nitric oxide is an important mediator for tumoricidal activity in vivo. Proc Natl Acad Sci U S A 91(20): 9407–9411.
Feun, L., and N. Savaraj. 2006. Pegylated arginine deiminase: A novel anticancer enzyme agent. Expert Opinion on Investigational Drugs 15(7): 815–822.
Gonzalez, G.G., and C.V. Byus. 1991. Effect of dietary arginine restriction upon ornithine and polyamine metabolism during two-stage epidermal carcinogenesis in the mouse. Cancer Research 51(11): 2932–2939.
Green, S.M., E. Eisenstein, P. McPhie, and P. Hensley. 1990. The purification and characterization of arginase from Saccharomyces cerevisiae. Journal of Biological Chemistry 265(3): 1601–1607.
Haki, G.D., and S.K. Rakshit. 2003. Developments in industrially important thermostable enzymes: A review. Bioresource technology 89(1): 17–34.
Hansen, M., and H.H. Hansen. 1989. Tumour markers in the clinical management of patients with small cell lung cancer. European Respiratory Journal 2(8): 700–701.
Hernandez, C.P., K. Morrow, L.A. Lopez-Barcons, J. Zabaleta, R. Sierra, C. Velasco, J. Cole, and P.C. Rodriguez. 2010. Pegylated arginase I: A potential therapeutic approach in T-ALL. Blood 115(25): 5214–5221.
Jenkins, D.C., I.G. Charles, L.L. Thomsen, D.W. Moss, L.S. Holmes, S.A. Baylis, P. Rhodes, K. Westmore, P.C. Emson, and S. Moncada. 1995. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A 92(10): 4392–4396.
Jenkinson, C.P., W.W. Grody, and S.D. Cederbaum. 1996. Comparative properties of arginases. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 114(1): 107–132.
Kanda, M., K. Ohgishi, T. Hanawa, and Y. Saito. 1997. Arginase of Bacillus brevis Nagano: Purification, properties, and implication in gramicidin S biosynthesis. Archives of Biochemistry and Biophysics 344(1): 37–42.
Kang, J.H., and Y.D. Cho. 1990. Purification and properties of Arginase from Soybean, Glycine max. Axes. Plant Physiol 93(3): 1230–1234.
Kelly, M.P., A.A. Jungbluth, B.W. Wu, J. Bomalaski, L.J. Old, and G. Ritter. 2012. Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. British Journal of Cancer 106(2): 324–332.
Kim, J.E., D.W. Jeong, and H.J. Lee. 2007. Expression, purification, and characterization of arginine deiminase from Lactococcus lactis ssp. lactis ATCC 7962 in Escherichia coli BL21. Protein Expression and Purification 53(1): 9–15.
Kotzia, G.A., K. Lappa, and N.E. Labrou. 2007. Tailoring structure-function properties of L-asparaginase: Engineering resistance to trypsin cleavage. Biochemical Journal 404(2): 337–343.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259): 680–685.
Liu, S., G.G. Pritchard, M.J. Hardman, and G.J. Pilone. 1995. Occurrence of arginine deiminase pathway enzymes in arginine catabolism by wine lactic acid bacteria. Applied and Environment Microbiology 61(1): 310–316.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193(1): 265–275.
Maheshwari, R., G. Bharadwaj, and M.K. Bhat. 2000. Thermophilic fungi: Their physiology and enzymes. Microbiology and Molecular Biology Reviews 64(3): 461–488.
Moore, S., D.H. Spackman, and W.H. Stein. 1958. Automatic recording apparatus for use in the chromatography of amino acids. Fed Proc 17(4): 1107–1115.
Moreno-Vivian, C., G. Soler, and F. Castillo. 1992. Arginine catabolism in the phototrophic bacterium Rhodobacter capsulatus E1F1. Purification and properties of arginase. European Journal of Biochemistry 204(2): 531–537.
Munder, M. 2009. Arginase: An emerging key player in the mammalian immune system. British Journal of Pharmacology 158(3): 638–651.
Patchett, M.L., R.M. Daniel, and H.W. Morgan. 1991. Characterisation of arginase from the extreme thermophile ‘Bacillus caldovelox’. Biochimica et Biophysica Acta 1077(3): 291–298.
Penninckx, M., J.P. Simon, and J.M. Wiame. 1974. Interaction between arginase and l-ornithine carbamoyltransferase in Saccharomyces cerevisiae. Purification of S. cerevisiae enzymes and evidence that these enzymes as well as rat-liver arginase are trimers. European Journal of Biochemistry 49(2): 429–442.
Philip, R., E. Campbell, and D.N. Wheatley. 2003. Arginine deprivation, growth inhibition and tumour cell death: 2. Enzymatic degradation of arginine in normal and malignant cell cultures. British Journal of Cancer 88(4): 613–623.
Schmidt, M.M., and R. Dringen. 2009. Differential effects of iodoacetamide and iodoacetate on glycolysis and glutathione metabolism of cultured astrocytes. Front Neuroenergetics 1: 1.
Simon, J.P., and V. Stalon. 1976. Purification and structure of arginase of Bacillus licheniformis. Biochimie 58(11–12): 1419–1421.
Singh, M., E. Silva, S. Schulze, D.A. Sinclair, K.A. Fitzpatrick, and B.M. Honda. 2000. Cloning and characterization of a new theta-class glutathione-S-transferase (GST) gene, gst-3, from Drosophila melanogaster. Gene 247(1–2): 167–173.
Sun, X., Z. Yang, S. Li, Y. Tan, N. Zhang, X. Wang, S. Yagi, T. Yoshioka, A. Takimoto, K. Mitsushima, A. Suginaka, E.P. Frenkel, and R.M. Hoffman. 2003. In vivo efficacy of recombinant methioninase is enhanced by the combination of polyethylene glycol conjugation and pyridoxal 5′-phosphate supplementation. Cancer Research 63(23): 8377–8383.
Viator, R.J., R.F. Rest, E. Hildebrandt, and D.J. McGee. 2008. Characterization of Bacillus anthracis arginase: Effects of pH, temperature, and cell viability on metal preference. BMC Biochemistry 9: 15.
Vieille, C., and G.J. Zeikus. 2001. Hyperthermophilic enzymes: Sources, uses, and molecular mechanisms for thermostability. Microbiology and Molecular Biology Reviews 65(1): 1–43.
Weickmann, J.L., M.E. Himmel, P.G. Squire, and D.E. Fahrney. 1978. Arginine deiminase from Mycoplasma arthritidis. Properties of the enzyme from log phase cultures. Journal of Biological Chemistry 253(17): 6010–6015.
Wu, G., and S. M. Morris Jr. 1998. Arginine metabolism: Nitric oxide and beyond. Biochemical Journal 336(Pt 1): 1–17.
Yang, Z., J. Wang, Q. Lu, J. Xu, Y. Kobayashi, T. Takakura, A. Takimoto, T. Yoshioka, C. Lian, C. Chen, D. Zhang, Y. Zhang, S. Li, X. Sun, Y. Tan, S. Yagi, E.P. Frenkel, and R.M. Hoffman. 2004a. PEGylation confers greatly extended half-life and attenuated immunogenicity to recombinant methioninase in primates. Cancer Research 64(18): 6673–6678.
Yang, Z., J. Wang, T. Yoshonlineioka, B. Li, Q. Lu, S. Li, X. Sun, Y. Tan, S. Yagi, E.P. Frenkel, and R.M. Hoffman. 2004b. Pharmacokinetics, methionine depletion, and antigenicity of recombinant methioninase in primates. Clinical Cancer Research 10(6): 2131–2138.
Zhang, J., X. Zhang, C. Wu, D. Lu, G. Guo, X. Mao, Y. Zhang, D.C. Wang, D. Li, and Q. Zou. 2011. Expression, purification and characterization of arginase from Helicobacter pylori in its apo form. PLoS ONE 6(10): e26205.
Acknowledgments
We appreciate the partial financial support by Zagazig University, Egypt, to Ashraf El-Sayed. We gratefully thank Prof. Sadik Esener, Nanoengineering Dep., University of California, San Diego, CA, USA, for his valuable discussion and revision of this work.
Conflict of interest
No conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.





Rights and permissions
About this article
Cite this article
El-Sayed, A.S., Shindia, A.A., Diab, A.A. et al. Purification and immobilization of l-arginase from thermotolerant Penicillium chrysogenum KJ185377.1; with unique kinetic properties as thermostable anticancer enzyme. Arch. Pharm. Res. (2014). https://doi.org/10.1007/s12272-014-0498-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12272-014-0498-y