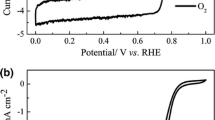
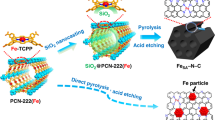
Abstract
Replacing Pt-based electrocatalysts for the oxygen reduction reaction (ORR) with high performance and low-cost non-precious metal catalysts is crucial for the commercialization of fuel cells. Herein, we present a highly efficient Fe-N-C porous ORR electrocatalyst with FeNxmoieties promoted by Fe2N nanoparticles derived from Fe-doped zeolitic imidazolate framework. The best-performing Fe-N-C/HPC-NH3catalyst exhibits a superior ORR activity with an onset (E0) and half-wave (E1/2) potential of 0.945 and 0.803 V (RHE), respectively, which is comparable to those of the commercial Pt/C in acidic media. Probing and acid-leaching experiments prove that FeNx moieties play an important role in the ORR and the Fe2N can further improve the ORR activity. Density functional theory calculation reveals a synergistic effect that the existence of Fe2N weakens the adsorption of ORR intermediates on active sites and lowers the reaction free energy of the potential limiting step, thus facilitating the ORR. Both experimental evidence and theoretical analysis for the enhancement of ORR activity by Fe2N decoration in Fe-N-C catalyst might inspire a new strategy for rational design of high performance non-precious metal catalysts.

Similar content being viewed by others
References
Debe, M. K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51.
Wu, G; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889.
Wang, Q.; Zhou, Z. Y.; Lai, Y. J.; You, Y.; Liu, J. G.; Wu, X. L.; Terefe, E.; Chen, C.; Song, L.; Rauf, M. et al. Phenylenediamine-based FeNx/C catalyst with high activity for oxygen reduction in acid medium and its active-site probing. J. Am. Chem. Soc. 2014, 136, 10882–10885.
Wang, Y. C.; Lai, Y. J.; Song, L.; Zhou, Z. Y.; Liu, J. G.; Wang, Q.; Yang, X. D.; Chen, C.; Shi, W.; Zheng, Y. P. et al. S-doping of an Fe/N/C ORR catalyst for polymer electrolyte membrane fuel cells with high power density. Angew. Chem., Int. Ed. 2015, 127, 10045–10048.
Yasuda, S.; Furuya, A.; Uchibori, Y.; Kim, J.; Murakoshi, K. Iron-nitrogen-doped vertically aligned carbon nanotube electrocatalyst for the oxygen reduction reaction. Adv. Funct. Mater. 2016, 26, 738–744.
Wu, G; Mack, N. H.; Gao, W.; Ma, S. G.; Zhong, R. Q.; Han, J. T.; Baldwin, J. K.; Zelenay, P. Nitrogen-doped graphene-rich catalysts derived from heteroatom polymers for oxygen reduction in nonaqueous Lithium-O2battery cathodes. ACS Nano 2012, 6, 9764–9776.
Lim, K. H.; Kim, H. Nitrogen-doped carbon catalysts derived from ionic liquids in the presence of transition metals for the oxygen reduction reaction. Appl. Catal. B: Environ. 2014, 158–159, 355–360.
Deng, D. H.; Yu, L.; Chen, X. Q.; Wang, G. X.; Jin, L.; Pan, X. L.; Deng, J.; Sun, G. Q.; Bao, X. H. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction. Angew. Chem., Int. Ed. 2013, 52, 371–375.
Zhang, Y.; Huang, L. B.; Jiang, W. J.; Zhang, X.; Chen, Y. Y.; Wei, Z. D.; Wan, L. J.; Hu, J. S. Sodium chloride-assisted green synthesis of a 3D Fe-N-C hybrid as a highly active electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 7781–7787.
Jiang, W. J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L. J.; Wang, J. Q.; Hu, J. S.; Wei, Z. D.; Wan, L. J. Understanding the high activity of Fe-N-C electrocatalysts in oxygen reduction: Fe/Fe3C nanoparticles boost the activity of Fe-Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578.
Deng, D. H.; Chen, X. Q.; Yu, L.; Wu, X.; Liu, Q. F.; Liu, Y.; Yang, H. X.; Tian, H. F.; Hu, Y. F.; Du, P. P. et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci. Adv. 2015, 1, e1500462.
Yang, X. D.; Zheng, Y. P.; Yang, J.; Shi, W.; Zhong, J. H.; Zhang, C. K.; Zhang, X.; Hong, Y. H.; Peng, X. X.; Zhou, Z. Y. et al. Modeling Fe/N/C catalysts in monolayer graphene. ACS Catal. 2017, 7, 139–145.
Chung, H. T.; Cullen, D. A.; Higgins, D.; Sneed, B. T.; Holby, E. F.; More, K. L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484.
Sun, T.; Jiang, Y. F.; Wu, Q.; Du, L. Y.; Zhang, Z. Q.; Yang, L. J.; Wang, X. Z.; Hu, Z. Is iron nitride or carbide highly active for oxygen reduction reaction in acidic medium? Catal. Sci. Technol. 2017, 7, 51–55.
Wang, M.; Yang, Y. S.; Liu, X. B.; Pu, Z. H.; Kou, Z. K.; Zhu, P. P.; Mu, S. C. The role of iron nitrides in the Fe-N-C catalysis system towards the oxygen reduction reaction. Nanoscale 2017, 9, 7641–7649.
Wang, L.; Yin, J.; Zhao, L.; Tian, C. G.; Yu, P.; Wang, J. Q.; Fu, H. G. Ion-exchanged route synthesis of Fe2N-N-doped graphitic nanocarbons composite as advanced oxygen reduction electrocatalyst. Chem. Commun. 2013, 49, 3022–3024.
Xiao, J. W.; Xu, Y. Y.; Xia, Y. T.; Xi, J. B.; Wang, S. Ultra-small Fe2N nanocrystals embedded into mesoporous nitrogen-doped graphitic carbon spheres as a highly active, stable, and methanol-tolerant electrocatalyst for the oxygen reduction reaction. Nano Energy 2016, 24, 121–129.
Kramm, U. I.; Herrmann-Geppert, I.; Bogdanoff, P.; Fiechter, S. Effect of an ammonia treatment on structure, composition, and oxygen reduction reaction activity of Fe-N-C catalysts. J. Phys. Chem. C 2011, 115, 23417–23427.
Meng, H.; Larouche, N.; Lefèvre, M.; Jaouen, F.; Stansfield, B.; Dodelet, J. P. Iron porphyrin-based cathode catalysts for polymer electrolyte membrane fuel cells: Effect of NH3 and Ar mixtures as pyrolysis gases on catalytic activity and stability. Electrochim. Acta 2010, 55, 6450–6461.
Liu, P.; Cheng, Q. Q.; Chen, C.; Zou, L. L.; Zou, Z. Q.; Yang, H. Preparation and oxygen reduction reaction catalytic performance of Fe, N co-doped carbon nanofibers with encapsulated iron nitride. Chem. J. Chin. Univ. 2018, 39, 2492–2499.
Deng, Y. J.; Dong, Y. Y.; Wang, G. H.; Sun, K. L.; Shi, X. D.; Zheng, L.; Li, X. H.; Liao, S. J. Well-defined ZIF-derived Fe-N codoped carbon nanoframes as efficient oxygen reduction catalysts. ACS Appl. Mater. Interfaces 2017, 9, 9699–9709.
Xiao, M. L.; Zhu, J. B.; Ma, L.; Jin, Z.; Ge, J. J.; Deng, X.; Hou, Y.; He, Q. G.; Li, J. K.; Jia, Q. Y. et al. Microporous framework induced synthesis of single-atom dispersed Fe-N-C acidic ORR catalyst and its in situ reduced Fe-N4 active site identification revealed by X-ray absorption spectroscopy. ACS Catal. 2018, 8, 2824–2832.
Chen, Y. J.; Ji, S. F.; Wang, Y. G.; Dong, J. C.; Chen, W. X.; Li, Z.; Shen, R. G.; Zheng, L. R.; Zhuang, Z. B.; Wang, D. S. et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2017, 56, 6937–6941.
Zhao, R.; Xia, W.; Lin, C.; Sun, J. L.; Mahmood, A.; Wang, Q. F.; Qiu, B.; Tabassum, H.; Zou, R. Q. A pore-expansion strategy to synthesize hierarchically porous carbon derived from metal-organic framework for enhanced oxygen reduction. Carbon 2017, 114, 284–290.
Gupta, S.; Zhao, S.; Ogoke, O.; Lin, Y.; Xu, H.; Wu, G. Engineering favorable morphology and structure of Fe-N-C oxygen-reduction catalysts through tuning of nitrogen/carbon precursors. ChemSusChem 2017, 10, 774–785.
Jaouen, F.; Herranz, J.; Lefèvre, M.; Dodelet, J. P.; Kramm, U. I.; Herrmann, I.; Bogdanoff, P.; Maruyama, J.; Nagaoka, T.; Garsuch, A. et al. Cross-laboratory experimental study of non-noble-metal electrocatalysts for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2009, 1, 1623–1639.
Jaouen, F.; Lefèvre, M.; Dodelet, J. P.; Cai, M. Heat-treated Fe/N/C catalysts for O2 electroreduction: Are active sites hosted in micropores? J. Phys. Chem. B 2006, 110, 5553–5558.
Zeng, S. S.; Lyu, F. C.; Nie, H. J.; Zhan, Y. W.; Bian, H. D.; Tian, Y. Y.; Li, Z.; Wang, A. W.; Lu, J.; Li, Y. Y. Facile fabrication of N/S-doped carbon nanotubes with Fe3O4 nanocrystals enchased for lasting synergy as efficient oxygen reduction catalysts. J. Mater. Chem. A 2017, 5, 13189–13195.
Li, G. N.; Zhang, J. J.; Li, W. S.; Fan, K.; Xu, C. J. 3D interconnected hierarchical porous N-doped carbon constructed by flake-like nanostructure with Fe/Fe3C for efficient oxygen reduction reaction and supercapacitor. Nanoscale 2018, 10, 9252–9260.
Peng, H. L.; Mo, Z. Y.; Liao, S. J.; Liang, H. G.; Yang, L. J.; Luo, F.; Song, H. Y.; Zhong, Y. L.; Zhang, B. Q. High performance Fe- and N-doped carbon catalyst with graphene structure for oxygen reduction. Sci. Rep. 2013, 3, 1765.
Lin, L.; Zhu, Q.; Xu, A. W. Noble-metal-free Fe-N/C catalyst for highly efficient oxygen reduction reaction under both alkaline and acidic conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033.
Wu, Z. Y.; Xu, X. X.; Hu, B. C.; Liang, H. W.; Lin, Y.; Chen, L. F.; Yu, S. H. Iron carbide nanoparticles encapsulated in mesoporous Fe-N-doped carbon nanofibers for efficient electrocatalysis. Angew. Chem., Int. Ed. 2015, 127, 8297–8301.
Liu, X.; Chen, C.; Cheng, Q. Q.; Zou, L. L.; Zou, Z. Q.; Yang, H. Binary nitrogen precursor-derived porous Fe-N-S/C catalyst for efficient oxygen reduction reaction in a Zn-air battery. Catalysts 2018, 8, 158.
Choi, I. A.; Kwak, D. H.; Han, S. B.; Park, J. Y.; Park, H. S.; Ma, K. B.; Kim, D. H.; Won, J. E.; Park, K. W. Doped porous carbon nanostructures as non-precious metal catalysts prepared by amino acid glycine for oxygen reduction reaction. Appl. Catal. B: Environ. 2017, 211, 235–244.
Wang, J. P.; Han, G. K.; Wang, L. G.; Du, L.; Chen, G. Y.; Gao, Y. Z.; Ma, Y. L.; Du, C. Y.; Cheng, X. Q.; Zuo, P. J. et al. ZIF-8 with ferrocene encapsulated: A promising precursor to single-atom Fe embedded nitrogen-doped carbon as highly efficient catalyst for oxygen electroreduction. Small 2018, 14, 1704282.
Cheng, Q. Q.; Mao, K.; Ma, L. S.; Yang, L. J.; Zou, L. L.; Zou, Z. Q.; Hu, Z.; Yang, H. Encapsulation of iron nitride by Fe-N-C shell enabling highly efficient electroreduction of CO2 to CO. ACS Energy Lett. 2018, 3, 1205–1211.
Liang, W.; Chen, J. X.; Liu, Y. W.; Chen, S. L. Density-functional-theory calculation analysis of active sites for four-electron reduction of O2 on Fe/N-doped graphene. ACS Catal. 2014, 4, 4170–4177.
Xiao, H.; Shao, Z. G.; Zhang, G.; Gao, Y.; Lu, W. T.; Yi, B. L. Fe-N-carbon black for the oxygen reduction reaction in sulfuric acid. Carbon 2013, 57, 443–451.
Wang, T.; Chen, Z. X.; Chen, Y. G.; Yang, L. J.; Yang, X. D.; Ye, J. Y.; Xia, H. P.; Zhou, Z. Y.; Sun, S. G. Identifying the active site of N-doped graphene for oxygen reduction by selective chemical modification. ACS Energy Lett. 2018, 3, 986–991.
Guo, D. H.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365.
Cheng, Q. Q.; Han, S. B.; Mao, K.; Chen, C.; Yang, L. J.; Zou, Z. Q.; Gu, M.; Hu, Z.; Yang, H. Co nanoparticle embedded in atomically-dispersed Co-N-C nanofibers for oxygen reduction with high activity and remarkable durability. Nano Energy 2018, 52, 485–493.
Xuan, C. J.; Hou, B. S.; Xia, W. W.; Peng, Z. K.; Shen, T.; Xin, H. L.; Zhang, G. A.; Wang, D. L. From a ZIF-8 polyhedron to three-dimensional nitrogen doped hierarchical porous carbon: An efficient electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 10731–10739.
Wang, R. W.; Yan, T. T.; Han, L. P.; Chen, G. R.; Li, H. R.; Zhang, J. P.; Shi, L. Y.; Zhang, D. S. Tuning the dimensions and structures of nitrogen-doped carbon nanomaterials derived from sacrificial g-C3N4/metal-organic frameworks for enhanced electrocatalytic oxygen reduction. J. Mater. Chem. A 2018, 6, 5752–5761.
Wan, X.; Wang, H. J.; Yu, H.; Peng, F. Highly uniform and monodisperse carbon nanospheres enriched with cobalt-nitrogen active sites as a potential oxygen reduction electrocatalyst. J. Power Sources 2017, 346, 80–88.
Yang, W. X.; Liu, X. J.; Chen, L. L.; Liang, L.; Jia, J. B. A metal-organic framework devised Co-N doped carbon microsphere/nanofiber hybrid as a free-standing 3D oxygen catalyst. Chem. Commun. 2017, 53, 4034–4037.
Wang, Z. H.; Jin, H. H.; Meng, T.; Liao, K.; Meng, W. Q.; Yang, J. L.; He, D. P.; Xiong, Y. L.; Mu, S. C. Fe, Cu-coordinated ZIF-derived carbon framework for efficient oxygen reduction reaction and zinc-air batteries. Adv. Funct. Mater. 2018, 28, 1802596.
Nandan, R.; Gautam, A.; Nanda, K. K. Maximizing the utilization of Fe-NjC/CN, centres for an air-cathode material and practical demonstration of metal-air batteries. J. Mater. Chem. A 2017, 5, 20252–20262.
Nerskov, J. K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J. R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892.
Jiang, Y. F.; Yang, L. J.; Sun, T.; Zhao, J.; Lyu, Z. Y.; Zhuo, O.; Wang, X. Z.; Wu, Q.; Ma, J.; Hu, Z. Significant contribution of intrinsic carbon defects to oxygen reduction activity. ACS Catal. 2015, 5, 6707–6712.
Kattel, S.; Wang, G. F. Reaction pathway for oxygen reduction on FeN4 embedded graphene. J. Phys. Chem. Lett. 2014, 5, 452–456.
Acknowledgements
The financial supports from the National Key Research and Development Program of China (No. 2017YFA0206500) and the National Natural Science Foundation of China (Nos. 21802161, 21673275, and 21533005) are greatly appreciated. We thank the HPC Platform of ShanghaiTech University for computing time.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Liu, X., Liu, H., Chen, C. et al. Fe2N nanoparticles boosting FeNx moieties for highly efficient oxygen reduction reaction in Fe-N-C porous catalyst. Nano Res. 12, 1651–1657 (2019). https://doi.org/10.1007/s12274-019-2415-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2415-7