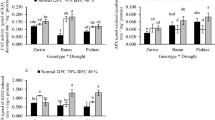
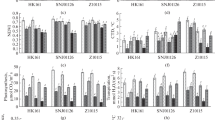
Abstract
Maize, a C4 sub-tropical crop, possesses higher temperature optima as compared to the C3 plants. Low temperature (LT) stress confines the growth and productivity of maize. In this context, two maize genotypes, LT tolerant Gurez local and LT susceptible Gujarat-Maize-6 (G-M-6) were analysed in present study for various osmolytes and gene expression of antioxidant enzymes including Ascorbate–glutathione (AsA-GSH) besides trehalose biosynthetic pathways. With the progressive LT treatment, Gurez local showed lesser accumulation of stress markers like hydrogen peroxide (H2O2) and malondialdehyde, a significant increase in osmoprotectants like free proline, total protein, total soluble sugars, trehalose, total phenolics and glycine betaine, and a significant reduction in the plant pigments as compared to the G-M-6. Additionally, Gurez local was found to possess a well-established antioxidant defense system as revealed from the elevated transcripts and enzyme activities of various enzymes of AsA-GSH pathway. Higher gene expression and enzyme activities were exhibited by superoxide dismutase, catalase and peroxidase besides the gene expression of trehalose biosynthetic pathway enzymes. Moreover, through principal component analyses, a positive correlation of all analysed parameters with the LT tolerance was noticed in Gurez local alone demarcating the genotypes on the basis of their extent of LT tolerance. Overall, the present study forms the basis for unravelling of LT tolerance mechanisms and improvement in the performance of the temperate maize.













Similar content being viewed by others
References
Albornoz K, Cantwell MI, Zhang L, Beckles DM (2019) Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress. Sci Rep 9:2795
Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP (2006) Protective role of antioxidant enzymes under high temperature stress. Plant Sci 171(3):382–388
Alves M, Chicau P, Matias H, Passarinho J, Pinheiro C, Ricardo CP (2011) Metabolic analysis revealed altered amino acid profiles in Lupinus albus organs as a result of boron deficiency. Physiol Plant 142:224–232
Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, Shahzad B, Zohaib A, Abbas F, Saleem MF, Ali I (2017) Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00069
Apak R, Güclü K, Özyürek M, Celik SE (2008) Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim Acta 160:413–419
Aras S, Eşitken A (2013) Effects of antifreeze proteins and glycine betaine on strawberry plants for resistance to cold temperature. Proc Intl Conf Agri Biotech 60:107–111
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf U, Kanu AS, Mo Z, Hussain S, Anjum SA, Khan I, Abbas RN, Tang X (2015) Lead toxicity in rice: effects, mechanisms, and mitigation strategies—a mini review. Env Sci Pol Res 22:18318–18332
Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, Wishart DS (2016) Heatmapper: web-enabled heat mapping for all. Nucleic Acid Res 44:W147–W153
Baek K-H, Skinner DZ (2012) Production of reactive oxygen species by freezing stress and the protective roles of antioxidant enzymes in plants. J Agri Chemi Environ 1:34
Bano S, Aslam M, Saleem M, Basra S, Aziz K (2015) Evaluation of maize accessions under low temperature stress at early growth stages. J Anim Plant Sci 25:392–400
Bartoli CG, Buet A, Grozeff GG, Galatro A, Simontacchi M (2017) Ascorbate-glutathione cycle and abiotic stress tolerance in plants. In: Hossain MA, Munné-Bosch S, Burritt DJ, Diaz-Vivancos P, Fujita M, Lorence A (eds) Ascorbic acid in plant growth development and stress tolerance. Springer, India, pp 177–200
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Batool A, Akram NA, Cheng Z-G, Lv G-C, Ashraf M, Afzal M, Xiong J-L, Wang J-Y, Xiong Y-C (2019) Physiological and biochemical responses of two spring wheat genotypes to non-hydraulic root-to-shoot signalling of partial and full root-zone drought stress. Plant Physiol Biochem 139:11–20
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytic Biochem 72:248–254
Cai H, Dong Y, Li Y, Li D, Peng C, Zhang Z, Wan X (2016) Physiological and cellular responses to fluoride stress in tea (Camellia sinensis) leaves. Acta Physiol Plant 38:144
Chandrasekar V, Sairam R, Srivastava G (2000) Physiological and biochemical responses of hexaploid and tetraploid wheat to drought stress. J Agr Crop Sci 185:219–227
Chaudhary DG, Chaudhary SR, Chaudhary MM, Mor VB (2017) Interaction effect of potassium and zinc on yield and nutrient uptake of forage maize (Zea mays L.) grown on loamy sand soil. Int J Chem Stud 5:1737–1739
Chen W, Li P, Chen T (2000) Glycinebetaine increases chilling tolerance and reduces chilling-induced lipid peroxidation in Zea mays L. Plant Cell Environ 23:609–618
de Azevedo Neto AD, Prisco JT, Enéas-Filho J, de Abreu CEB, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Cholakova-Bimbalova R, Vassilev A (2017) Effect of chilling response on the photosynthetic performance of young plants from maize (Zea mays) hybrids. CBU International Conference Proceedings, pp 1118–1123
Delorge I, Janiak M, Carpentier S, Van Dijck P (2014) Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants. Front Plant Sci 5:147
Oligosaccharides. Method Plant Biochem, Acedemic Press 2:189–218
Farooq M, Aziz T, Wahid A, Lee D-J, Siddique KH (2009) Chilling tolerance in maize: agronomic and physiological approaches. Crop Pasture Sci 60:501–516
Farooqi MQU, Lee JK (2016) Cold stress evaluation among maize (Zea mays L.) inbred lines in different temperature conditions. Plant Breed Biotechnol 4:352–361
Fu Y, Ma H, Chen S, Gu T, Gong J (2017) Control of proline accumulation under drought via a novel pathway comprising the histone methylase CAU1 and the transcription factor ANAC055. J Exp Bot 69:579–588
Grieve C, Grattan S (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Gu Y, He L, Zhao C, Wang F, Yan B, Gao Y, Li Z, Yang K, Xu J (2017) Biochemical and transcriptional regulation of membrane lipid metabolism in maize leaves under low temperature. Front Plant Sci 8:2053
Hakim M, Juraimi AS, Hanafi M, Ismail MR, Selamat A, Rafii M, Latif M (2014) Biochemical and anatomical changes and yield reduction in rice (Oryza sativa L.) under varied salinity regimes. BioMed Res Intnl 2014
Harb A, Awad D, Samarah N (2015) Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. J Plant Interact 10:109–116
Hodges DM, Andrews CJ, Johnson DA, Hamilton RI (1997) Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J Exp Bot 48:1105–1113
Hou P, Liu Y, Xie R, Ming B, Ma D, Li S, Mei X (2014) Temporal and spatial variation in accumulated temperature requirements of maize. Field Crop Res 158:55–64
Huo Y, Wang M, Wei Y, Xia Z (2016) Overexpression of the maize psbA gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense gene expression in tobacco. Front Plant Sci 6:1223
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L (2018) Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front Plant Sci 9:393
Iordachescu M, Imai R (2008) Trehalose biosynthesis in response to abiotic stresses. J Integ Plant Biol 50:1223–1229
Jan N, Majeed U, Andrabi KI, John R (2018) Cold stress modulates osmolytes and antioxidant system in Calendula officinalis. Acta Physiol Planta 40:73
Jan N, Qazi HA, Raja V, John R (2019) Proteomics: A tool to decipher cold tolerance. Theo and Exp Plant Physiol 31(1):183–213
Janmohammadi M (2013) Metabolomic analysis of low temperature responses in plants. Curr Opin Agri 1
Janowiak F, Luck E, Dörffling K (2003) Chilling tolerance of maize seedlings in the field during cold periods in spring is related to chilling-induced increase in abscisic acid level. J Agr Crop Sci 189:156–161
Kellős T (2008) Effect of abiotic stress on antioxidants in maize. Acta Biol Szegediensis 52:173–174
Kim S-I, Tai TH (2011) Evaluation of seedling cold tolerance in rice cultivars: a comparison of visual ratings and quantitative indicators of physiological changes. Euphytica 178:437–447
Kosmas SA, Argyrokastritis A, Loukas MG, Eliopoulos E, Tsakas S, Kaltsikes PJ (2006) Isolation and characterization of drought-related trehalose 6-phosphate-synthase gene from cultivated cotton (Gossypium hirsutum L.). Planta 223:329–339
Kovinich N, Kayanja G, Chanoca A, Otegui MS, Grotewold E (2015) Abiotic stresses induce different localizations of anthocyanins in arabidopsis. Plant Signal Behav 10:1027850
Król A, Amarowicz R, Weidner S (2015) The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves. J Plant Physiol 189:97–104
Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB, Biosciences JMI (2014) Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio Protoc 4:1108
Kumari R, Ashraf S, Bagri GK, Khatik SK, Bagri DK, Bagdi DL (2018) Extraction and estimation of chlorophyll content of seed treated lentil crop using DMSO and acetone. J Pharm Phytochem 7(3):249–250
Lee J-H, Oh M-M (2015) Short-term low temperature increases phenolic antioxidant levels in kale. Hort Environ Biotech 56:588–596
Li R-h, Guo P-g, Michael B, Stefania G, Salvatore C (2006) Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agri Sci China 5:751–757
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Limited 591–592
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Livingston DP, Hincha DK, Heyer AG (2009) Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol Life Sci 66:2007–2023
Longo V, Kamran RV, Michaletti A, Toorchi M, Zolla L, Rinalducci S (2017) Proteomic and physiological response of spring barley leaves to cold stress. Cell 6 (7)
Lu P, Sang WG, Ma KP (2008) Differential responses of the activities of antioxidant enzymes to thermal stresses between two invasive Eupatorium species in China. J Integ Plant Biol 50:393–401
Marocco A, Lorenzoni C, Francheboud Y (2005) Chilling stress in maize. Maydica 50:571
Mutlu S, Atıcı Ö, Nalbantoğlu B, Mete E (2016) Exogenous salicylic acid alleviates cold damage by regulating antioxidative system in two barley (Hordeum vulgare L.) cultivars. Front Life Sci 9:99–109
Naidu B, Paleg L, Aspinall D, Jennings A, Jones G (1991) Amino acid and glycine betaine accumulation in cold-stressed wheat seedlings. Phytochemistry 30:407–409
Nakata M, Ohme-Takagi M (2014) Quantification of anthocyanin content. Bio-Protocol 4(7):1098
Nunes C, Schluepmann H, Delatte TL, Wingler A, Silva AB, Fevereiro PS, Jansen M, Fiorani F, Wiese-Klinkenberg A, Paul MJ (2013) Regulation of growth by the trehalose pathway: relationship to temperature and sucrose. Plant Signal Behav 8:e26626
Pramanik MHR, Imai R (2005) Functional identification of a trehalose 6-phosphate phosphatase gene that is involved in transient induction of trehalose biosynthesis during chilling stress in rice. Plant Mol Biol 58:751–762
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6:65–74
Rajput RD, Patil RP (2017) The comparative study on spectrophotometric analysis of chlorophyll and carotenoids pigments from non-leguminous fodder crops. Int J Innov Sci Eng Tech 7:140–148
Ramazan S, Bhat HA, Zargar MA, Ahmad P, John R. (2021) Combined gas exchange characteristics, chlorophyll fluorescence and response curves as selection traits for temperature tolerance in maize genotypes. Photo Res 1–13
Revilla P, Rodríguez VM, Ordás A, Rincent R, Charcosset A, Giauffret C, Melchinger AE, Schön C-C, Bauer E, Altmann T (2014) Cold tolerance in two large maize inbred panels adapted to European climates. Crop Sci 54:1981–1991
Revilla P, Rodríguez VM, Ordás A, Rincent R, Charcosset A, Giauffret C, Melchinger AE, Schön C-C, Bauer E, Altmann T (2016) Association mapping for cold tolerance in two large maize inbred panels. BMC Plant Biol 16:127
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Sato Y, Murakami T, Funatsuki H, Matsuba S, Saruyama H, Tanida M (2001) Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. J Exp Bot 52:145–151
Selim S, Hassan YM, Saleh AM, Habeeb TH, AbdElgawad H (2019) Actinobacterium isolated from a semi-arid environment improves the drought tolerance in maize (Zea mays L.). Plant Physiol Biochem 142:15–21
Serraj R, Sinclair T (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ 25:333–341
Sharma I, Ching E, Saini S, Bhardwaj R, Pati PK (2013) Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem 69:17–26
Songstad D, Duncan D, Widholm J (1990) Proline and polyamine involvement in chilling tolerance of maize suspension cultures. J Exp Bot 41:289–294
Su X, Wei F, Huo Y, Xia Z (2017) Comparative physiological and molecular analyses of two contrasting flue-cured tobacco genotypes under progressive drought stress. Front Plant Sci 8:827
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Planta 126:45–51
Tao D-L, Öquist G, Wingsle G (1998) Active oxygen scavengers during cold acclimation of Scots pine seedlings in relation to freezing tolerance. Cryobiology 37:38–45
Theocharis A, Clément C, Barka EA (2012) Physiological and molecular changes in plants grown at low temperatures. Planta 235:1091–1105
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. An Rev Plant Biol 50:571–599
Turhan E, Ergin S (2012) Soluble sugars and sucrose-metabolizing enzymes related to cold acclimation of sweet cherry cultivars grafted on different rootstocks. Scient World J. https://doi.org/10.1100/2012/979682
Uarrota VG, Stefen DLV, Leolato LS, Gindri DM, Nerling D (2018) Revisiting carotenoids and their role in plant stress responses: from biosynthesis to plant signaling mechanisms during stress. In: Gupta DK, Palma JM, Corpas FJ (eds) Antioxidants and antioxidant enzymes in higher plants. Springer, pp 207–232
Wang W-B, Kim Y-H, Lee H-S, Kim K-Y, Deng X-P, Kwak S-S (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem 47:570–577
Wang X, Shan X, Wu Y, Su S, Li S, Liu H, Han J, Xue C, Yuan Y (2016) iTRAQ-based quantitative proteomic analysis reveals new metabolic pathways responding to chilling stress in maize seedlings. J Proteom 146:14–24
Wani MA, Jan N, Qazi HA, Andrabi KI, John R (2018) Cold stress induces biochemical changes, fatty acid profile, antioxidant system and gene expression in Capsella bursa pastoris L. Acta Physiol Plant 40:167
Wu A, Hammer GL, Doherty A, von Caemmerer S, Farquhar GD (2019) Quantifying impacts of enhancing photosynthesis on crop yield. Nature Plants 5:380–388
Xu S, Jiang Y, Cui W, Jin Q, Zhang Y, Bu D, Fu J, Wang R, Zhou F, Shen W (2017) Hydrogen enhances adaptation of rice seedlings to cold stress via the reestablishment of redox homeostasis mediated by miRNA expression. Plant Soil 414:53–67
Zhou J, Wang J, Shi K, Xia XJ, Zhou YH, Yu JQ (2012) Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol Biochem 60:141–149
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramazan, S., Qazi, H.A., Dar, Z.A. et al. Low temperature elicits differential biochemical and antioxidant responses in maize (Zea mays) genotypes with different susceptibility to low temperature stress. Physiol Mol Biol Plants 27, 1395–1412 (2021). https://doi.org/10.1007/s12298-021-01020-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-01020-3