Abstract
Purpose
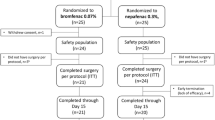
To compare the efficacy of bromfenac 0.09% and dexamethasone 0.1% in the treatment of anterior chamber inflammation after uncomplicated cataract surgery.
Methods
Seventy-six patients with senile cataracts and no other ocular comorbidities who underwent uneventful phacoemulsification were randomized 1:1 to receive dexamethasone ophthalmic suspension 0.1% or bromfenac ophthalmic solution 0.09% for 2 weeks. All patients were examined on the day before surgery and postoperatively at day 1, 3, 7, 9, 11, 14 and 30. Laser flare photometry was used to quantify anterior chamber inflammation and optical coherence tomography to measure macular thickness.
Results
Bromfenac was as effective as dexamethasone in reducing inflammation in the anterior chamber of the eye. Laser flare increased the day after surgery and progressively decreased after starting the treatment with no statistically significant difference between dexamethasone and bromfenac at all time points. Visual acuity improved steadily after surgery in both groups. Mean macular thickness was similar in both the dexamethasone and bromfenac arms at 1 month.
Conclusions
Short-term therapy with topical bromfenac alone is as effective as dexamethasone in low-risk cataract surgery patients.
Trial Registration
ClinicalTrials.gov # NCT03317847; EudraCT # 2016-004358-14.
Funding
Santa Maria Nuova Hospital IRCCS, Reggio Emilia, Italy.




Similar content being viewed by others
References
Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390(10094):600–12.
Shalam KV (2010-2011) Ocular pharmacotherapeutics. In: American Academy of Ophthalmology, Basic and Clinical Science Course. Fundamentals and principles of ophthalmology, p. 358.
Kessel L, Tendal B, Jørgensen KJ, et al. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: a systematic review. Ophthalmology. 2014;121(10):1915–24.
Tsangaridou MA, Grzybowski A, Gundlach E, Pleyer U. Controversies in NSAIDs use in cataract surgery. Curr Pharm Des. 2015;21(32):4707–17.
Duan P, Liu Y, Li J. The comparative efficacy and safety of topical non-steroidal anti-inflammatory drugs for the treatment of anterior chamber inflammation after cataract surgery: a systematic review and network meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):639–49.
Boscia F, Giancipoli E, D’Amico Ricci G, Pinna A. Management of macular oedema in diabetic patients undergoing cataract surgery. Curr Opin Ophthalmol. 2017;28(1):23–8.
Wielders LH, Schouten JS, Aberle MR, et al. Treatment of cystoid macular edema after cataract surgery. J Cataract Refract Surg. 2017;43(2):276–84.
Wielders LHP, Schouten JSAG, Nuijts RMMA. Prevention of macular edema after cataract surgery. Curr Opin Ophthalmol. 2018;29(1):48–53.
Lim BX, Lim CH, Lim DK, Evans JR, Bunce C, Wormald R. Prophylactic non-steroidal anti-inflammatory drugs for the prevention of macular oedema after cataract surgery. Cochrane Database Syst Rev. 2016;11:CD006683.
Hoffman RS, Braga-Mele R, Donaldson K, et al. Cataract surgery and nonsteroidal antiinflammatory drugs. J Cataract Refract Surg. 2016;42(9):1368–79.
Ahmed M, Stephen Foster C. Steroid therapy for ocular inflammatory disease. Focal Points Clin Modul Ophthalmol. 2006;24(7):1–7.
Kim SJ, Schoenberger SD, Thorne JE, Ehlers JP, Yeh S, Bakri SJ. Topical nonsteroidal anti-inflammatory drugs and cataract surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(11):2159–68.
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16.
Kempen JH, Ganesh SK, Sangwan VS, Rathinam SR. Interobserver agreement in grading activity and site of inflammation in eyes of patients with uveitis. Am J Ophthalmol. 2008;146(6):813.e1–818.e1.
Sawa M, Tsurimaki Y, Tsuru T, Shimizu H. New quantitative method to determine protein concentration and cell number in aqueous in vivo. Jpn J Ophthalmol. 1988;32(2):132–42.
Tugal-Tutkun I, Herbort CP. Laser flare photometry: a noninvasive, objective, and quantitative method to measure intraocular inflammation. Int Ophthalmol. 2010;30(5):453–64.
Chylack LT Jr, Wolfe JK, Singer DM, et al. The lens opacities classification system III. The longitudinal study of cataract study group. Arch Ophthalmol. 1993;111:831–6.
Donnenfeld ED, Holland EJ, Stewart RH, Gow JA, Grillone LR. Bromfenac ophthalmic solution 0.09% (Xibrom) study group. Bromfenac ophthalmic solution 0.09% (Xibrom) for postoperative ocular pain and inflammation. Ophthalmology. 2007;114:1653–62.
Coassin M, Iovieno A, Soldani A, et al. Bromfenac ophthalmic solution 0.09% as an adjunctive therapy to topical steroids after cataract surgery in pseudoexfoliation syndrome. J Cataract Refract Surg. 2016;42(8):1119–25.
Juthani VV, Clearfield E, Chuck RS. Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery. Cochrane Database Syst Rev. 2017;7:CD010516.
Ursell PG, Spalton DJ, Whitcup SM, Nussenblatt RB. Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg. 1999;25:1492–7.
Loewenstein A, Zur D. Postsurgical cystoid macular edema. Dev Ophthalmol. 2010;47:148–59.
Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557–634.
Wittpenn JR, Silverstein S, Heier J, Kenyon KR, Hunkeler JD, Earl M. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol. 2008;146:554–60.
Wielders LH, Lambermont VA, Schouten JS, et al. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta-analysis. Am J Ophthalmol. 2015;160(968–981):e33.
Wielders LHP, Schouten JSAG, Winkens B, et al. European multicenter trial of the prevention of cystoid macular edema after cataract surgery in nondiabetics: ESCRS PREMED study report 1. J Cataract Refract Surg. 2018;44(4):429–39.
Wielders LHP, Schouten JSAG, Winkens B, et al. Randomized controlled European multicenter trial on the prevention of cystoid macular edema after cataract surgery in diabetics: ESCRS PREMED Study Report 2. J Cataract Refract Surg. 2018;44(7):836–47.
Acknowledgements
The authors thank all patients for kindly participating in the study.
Funding
The study was sponsored by the Santa Maria Nuova Hospital IRCCS, Reggio Emilia, Italy, who also funded the Rapid Service Fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure
Marco Coassin, Michele De Maria, Valentina Mastrofilippo, Luca Braglia, Luca Cimino, Antonio Sartori and Luigi Fontana have nothing to declare.
Compliance with Ethics Guidelines
The study protocol was approved by the local ethics committee (Comitato Etico dell’Area Vasta Emilia Nord) and the trial was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9693353.
Rights and permissions
About this article
Cite this article
Coassin, M., De Maria, M., Mastrofilippo, V. et al. Anterior Chamber Inflammation After Cataract Surgery: A Randomized Clinical Trial Comparing Bromfenac 0.09% to Dexamethasone 0.1%. Adv Ther 36, 2712–2722 (2019). https://doi.org/10.1007/s12325-019-01076-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01076-4