Abstract
Introduction
The ALCYONE trial found that daratumumab in combination with bortezomib, melphalan, and prednisone (D-VMP) can significantly improve progression-free survival (PFS) and overall survival (OS) for patients with transplant-ineligible, newly diagnosed multiple myeloma (MM) in China. In the present study, we evaluated the cost-effectiveness of D-VMP versus VMP for patients with newly diagnosed MM in China.
Methods
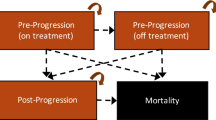
A Markov model was used to estimate the cost-effectiveness of frontline D-VMP versus VMP for MM. The life years (LYs), quality-adjusted LYs (QALYs), and incremental cost-effectiveness ratio (ICER) were calculated. A series of sensitivity analyses was performed to assess the robustness of the model and address uncertainties in variable estimates. Subgroup analysis was also performed.
Results
D-VMP provided an additional 2.99 LYs and 1.67 QALYs compared with VMP, with incremental $64,920 per LY and $116,015 per QALY gained. The results of the univariable sensitivity analysis showed that the parameter that had the greatest impact on the ICER was the cost of subsequent treatment and daratumumab. When the cost of daratumumab was 100%, 70%, 50%, and 30% of the current price, the probability of D-VMP being cost-effective was 2.49%, 16.11%, 39.09%, and 70.73% at the willingness-to-pay (WTP) threshold of $30,950/QALY, respectively. The results demonstrated that the ICER in all subgroups remained > $30,950/QALY.
Conclusion
D-VMP versus VMP is likely to exceed the commonly accepted values of cost-effectiveness in patients with transplant-ineligible, newly diagnosed MM in China.


Similar content being viewed by others
References
Wang S, Xu L, Feng J, et al. Prevalence and incidence of multiple myeloma in urban area in China: a national population-based analysis. Front Oncol. 2019;9:1513.
International Agency for Reseach on Cancer. Estimated number of new cases in 2018, CHina, both sexes, all age. 2020. http://globocan.iarc.fr. Accessed 01 Sep 2020.
Usmani SZ, Cavenagh JD, Belch AR, et al. Cost-effectiveness of lenalidomide plus dexamethasone vs. bortezomib plus melphalan and prednisone in transplant-ineligible US patients with newly-diagnosed multiple myeloma. J Med Econ. 2016;19(3):243–58.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: multiple myeloma version 2.2019. 2018. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed 01 Sept 2020.
Mohty M, Harousseau JL. Treatment of autologous stem cell transplant-eligible multiple myeloma patients: ten questions and answers. Haematologica. 2014;99(3):408–16.
Naymagon L, Abdul-Hay M. Novel agents in the treatment of multiple myeloma: a review about the future. J Hematol Oncol. 2016;9(1):52.
Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–28.
Mateos MV, Cavo M, Blade J, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395(10218):132–41.
Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104–15.
Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139.
World Health Organization. Global Health Observatory data repository: Life tables by country China. 2020. https://apps.who.int/gho/data/view.main.60340. Accessed 12 Aug 2020.
Lu S, Yu Y, Fu S, Ren H. Cost-effectiveness of ALK testing and first-line crizotinib therapy for non-small-cell lung cancer in China. PLoS ONE. 2018;13(10):e0205827.
Cai H, Zhang L, Li N, Zheng B, Liu M. Cost-effectiveness analysis on binary/triple therapy on the basis of ixazomib or bortezomib for refractory or relapsed multiple myeloma. Leuk Lymphoma. 2019;60(12):2951–9.
Ailawadhi S, DerSarkissian M, Duh MS, et al. Cost offsets in the treatment journeys of patients with relapsed/refractory multiple myeloma. Clin Ther. 2019;41(3):477–93.
Pelligra CG, Parikh K, Guo S, et al. Cost-effectiveness of pomalidomide, carfilzomib, and daratumumab for the treatment of patients with heavily pretreated relapsed-refractory multiple myeloma in the United States. Clin Ther. 2017;39(10):1986-2005.e1985.
Gu X, Zhang Q, Chu YB, et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer. 2019;127:84–9.
You R, Liu J, Wu DB, et al. Cost-effectiveness analysis of EGFR mutation testing and afatinib versus gemcitabine-cisplatin as first-line therapy for advanced non-small-cell lung cancer in China. Cancer management and research. 2019;11:10239–48.
Maurer KA, Chen HF, Wagner AL, et al. Cost-effectiveness analysis of pneumococcal vaccination for infants in China. Vaccine. 2016;34(50):6343–9.
Li S, Peng L, Tan C, et al. Cost-Effectiveness of ramucirumab plus paclitaxel as a second-line therapy for advanced gastric or gastro-oesophageal cancer in China. PLoS ONE. 2020;15(5):e0232240.
Zeng X, Peng L, Peng Y, Tan C, Wan X. Economic evaluation of adding daratumumab to a regimen of bortezomib + dexamethasone in relapsed or refractory multiple myeloma: based on the latest updated analysis of CASTOR. Clin Ther. 2020;42(2):251–262255.
Kumar SK, Callander NS, Adekola K, et al. Multiple myeloma, version 3.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(12):1685–717.
Hoyle M, Green C, Thompson-Coon J, et al. Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health. 2010;13(1):61–8.
Zhang TT, Wang S, Wan N, Zhang L, Zhang Z, Jiang J. Cost-effectiveness of daratumumab-based triplet therapies in patients with relapsed or refractory multiple myeloma. Clin Ther. 2018;40(7):1122–39.
Carlson JJ, Guzauskas GF, Chapman RH, et al. Cost-effectiveness of drugs to treat relapsed/refractory multiple myeloma in the United States. J Manag Care Spec Pharm. 2018;24(1):29–38.
Gong CL, Studdert AL, Liedtke M. Daratumumab vs pomalidomide for the treatment of relapsed/refractory multiple myeloma: a cost-effectiveness analysis. Am J Hematol. 2019;94(3):E68–70.
Patel KK, Giri S, Parker TL, Bar N, Neparidze N, Huntington SF. Cost-effectiveness of first-line versus second-line use of daratumumab in older, transplant-ineligible patients with multiple myeloma. J Clin Oncol. 2021. https://doi.org/10.1200/JCO.20.01849.
China National Medical Security Bureau. The National Medical Insurance Administration and the Ministry of Human Resources and Social Security have published the list of medicines for national medical insurance negotiation. 2019. http://www.nhsa.gov.cn/art/2019/11/28/art_14_2052.html. Accessed 20 Jan 2021.
Wan X, Peng L, Li Y. A review and comparison of methods for recreating individual patient data from published Kaplan-Meier survival curves for economic evaluations: a simulation study. PLoS ONE. 2015;10(3):e0121353.
Acknowledgements
Funding
The work and the journal’s Rapid Service Fee were supported by grants from the National Natural Science Foundation of China (grant numbers: 81401547, 81603081, 82073818, and 71874209), the Key Science-Technology Research and Development Program of Hunan Province (grant number: 2016JC2062 and 2020JJ8046), and the Hunan Provincial Natural Science Foundation of China (grant number: 2019JJ40411).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Xiaohui Zeng, QiaoLiu, Liubao Peng, Ye Peng, Lidan Yi, Xia Luo, Sini Li, Xiaomin Wan, and Chongqing Tan have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on a retrospective study and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, X., Liu, Q., Peng, L. et al. Cost-Effectiveness Analysis of Adding Daratumumab to a Regimen of Bortezomib, Melphalan, and Prednisone in Newly Diagnosed Multiple Myeloma. Adv Ther 38, 2379–2390 (2021). https://doi.org/10.1007/s12325-021-01699-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01699-6