Abstract
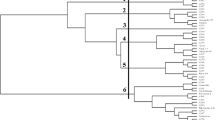
Molecular markers could be used to screen for somaclonal variation during the process of genetic transformation. We aimed to test target region amplified polymorphism (TRAP) marker to quickly predict similarity to the parental line of different transgenic lines. DNA of transgenic lines, wild-type genotypes and additional sugarcane clones was genotyped with TRAP markers. Amplification products were separated by electrophoresis on polyacrylamide denaturing gels, images were analyzed, and bands were transformed into a 0/1 matrix. Similarity was calculated using the Jaccard coefficient and dendrograms generated using UPGMA analysis. TRAP was initially used to determine whether the close growth resemblance between six herbicide-tolerant lines and their parental cultivar RA87-3 was also true at the genetic level. The genetic characterization confirmed the preliminary phenotypic evaluations since transformed lines exhibited none or only minor genetic changes whereas lines with growth aberrations also included in the analysis showed a significant degree of polymorphism. The incorporation of other genotypes as controls allowed us to internally evaluate the accuracy of the survey ensuring that a significant number of polymorphic bands were analyzed. These markers were routinely applied to evaluate transgenic lines of LCP85-384, TUCCP77-42, TUC95-10 and TUC03-12 at early stages of the characterization process. Our results showed that the use of TRAP markers is a rapid and recommendable first approach to identify transformed plants that are genetically close to their parental genotype as they could be applied at the early stages of evaluation to select for the most valuable lines to carry out field tests.

Similar content being viewed by others
References
Aljanabi, Shadan Muslin, Luc Forget, and Asha Dookun. 1999. An improved and rapid protocol for the isolation of polysaccharide and polyphenol free sugarcane DNA. Plant Molecular Biology Report 17: 281.
Alwala, Sreddhar, Andru Suman, Jie Arro, John Veremis, and Collins A. Kimbeng. 2006. Target region amplification polymorphism (TRAP) for assessing genetic diversity in sugarcane germplasm collections. Crop Science 46: 448–455.
Arencibia, Ariel D., Elva R. Carmona, T. Cornide María, Stefan Castiglione, José O’Relly, Antonio Chinea, Pedro Oramas, and Francesco Sala. 1999. Somaclonal variation in insect -resistant transgenic sugarcane (Saccharum hybrid) plants produced by cell electroporation. Transgenic Research 8: 349–360.
Carmona, Elva R., Ariel D. Arencibia, Julio A. López, D. June Simpson, and Francesco Sala Vargas. 2005. Analysis of genomic variability in transgenic sugarcane plants produced by Agrobacterium tumefaciens infection. Plant Breeding 124: 33–38.
Creste, Silvana, Klauss A.G. Accoroni, Luciana Rossini Pinto, Roland Vencovsky, Marcos A. Gimenes, Mauro A. Xavier, and Marcos G.A. Landell. 2010. Genetic variability among sugarcane genotypes based on polymorphisms in sucrose metabolism and drought tolerance genes. Euphytica 172: 435–446.
Di Rienzo, Julio, Fernando Casanoves, Mónica Balzarini, Laura González, Margot Tablada, and Carlos Robledo. 2009. InfoStat. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
Gilbert, Robert, Glynn Neil, Jack Comstock, and Michael J. Davis. 2009. Agronomic performance and genetic characterization of sugarcane transformed for resistance to sugarcane yellow leaf virus. Field Crops Research 111: 39–46.
Joyce, Priya, Scott Hermann, Anthony O'Connell, Quang Dinh, Leonard Shumbe, and Prakash Lakshmanan. 2014. Field performance of transgenic sugarcane produced using Agrobacterium and biolistic methods. Plant Biotechnology Journal 12: 411–424.
Li, Guofand, and Carlos F. Quiros. 2001. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theoretical and Applied Genetics 103: 455–461.
Perera, María F., Marta E. Arias, Diego Costilla, Ana C. Luque, María B. García, Carolina Díaz Romero, Josefina Racedo, Santiago Ostengo, María P. Filippone, María I. Cuenya, and Atilio P. Castagnaro. 2012. Genetic diversity assessment and genotype identification in sugarcane based on DNA markers and morphological traits. Euphytica 185: 491–510.
Sneath, Peter H., and Robert R. Sokal. 1973. Numerical taxonomy. The principles and practice of numerical classification. San Francisco: Freeman.
Taparia, Yogesh, María Gallo, and Fredy Altpeter. 2012. Comparison of direct and indirect embryogenesis protocols, biolistic gene transfer and selection parameters for efficient genetic transformation of sugarcane. Plant Cell, Tissue and Organ Culture 111: 131–141.
Taylor, Paul W.J., Tracy A. Fraser, Hian-Lein Ko, and Robert J. Henry. 1995. RAPD analysis of sugarcane during tissue culture. In Current issues in plant molecular and cellular biology, ed. Mario Terzi, Rino Cella, and A. Falavigna, 241–246. Dordrecht: Kluwer Academic.
Union for the Protection of New Varieties of Plants (UPOV). 2005. Directrices para la ejecución del examen de la distinción, la homogeneidad y la estabilidad tg/186/1. www.Upov.Int/es/publications/tg-rom/tg186/tg_186_1.Pdf. Accessed on June 2014.
Acknowledgements
This project was supported by EEAOC, CONICET and PICTO 2016-0120 from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by María Francisca Perera, Silvia Natalia Ovejero and Josefina Racedo. The first draft of the manuscript was written by María Francisca Perera and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest related to the work described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perera, M.F., Ovejero, S.N., Racedo, J. et al. TRAP Markers Allow the Identification of Transgenic Lines that are Genetically Close to Their Parental Genotype. Sugar Tech 22, 750–755 (2020). https://doi.org/10.1007/s12355-020-00836-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-020-00836-9