Abstract
A multilayered TiAlSiN thin film consisting of alternating nanocrystalline Ti(Si)N and Al(Si)N nanolayers was deposited on steel by arc ion plating. The film composition was 26Ti–16.3Al–1.2Si–56.50N in at%. The film was corroded at 800–1000 °C for 4–100 h in N2/0.1%H2S gas to study its corrosion behavior in hostile (H, S)-containing environments. The corrosion was primarily governed by oxidation, because oxides of Ti and Al were much more stable than the corresponding sulfides. The oxygen source for oxidation was impurity oxygen in N2/0.1%H2S gas. Initially, a superficial Al2O3 scale formed. Soon, the scale developed into the outer TiO2-rich layer and the inner Al2O3-rich layer, beneath which formed an oxygen affected zone. As corrosion progressed, Si tended to accumulate in the lower part of the inner Al2O3-rich layer owing to its thermodynamic nobility. Preferential oxidation of Al to Al2O3, formation of fine, dense Al2O3 and TiO2 grains in the oxide scale, and strong Ti–Si, Al–N and Ti–N bonds in the TiAlSiN film caused the scale to grow quite slowly and suppressed fast inward diffusion of sulfur and hydrogen as well as fast outward diffusion of Ti, Al, and Si. Therefore, the film displayed good corrosion resistance at 800–900 °C for up to 100 h. However, it corroded completely, with partial scale spallation and whisker growth at 1000 °C for 50 h.
Graphic Abstract

Similar content being viewed by others
1 Introduction
Wear-resistant, hard TiN film is widely used to improve the performance and service life of cutting tools and die molds [1, 2]. However, its mechanical properties and thermal stability are insufficient for rigorous machining operations requiring high speed cutting, high feed rate, and dry cutting [3]. Hence, substitutional elements such as Al and Si are added to fcc-TiN films for advanced tribological applications. These TiAlSiN films displayed improved hardness [4,5,6,7,8], cutting performance [9, 10], wear resistance [11, 12], corrosion resistance [13], thermal stability [14], and oxidation resistance [15,16,17,18,19,20,21,22,23]. They have been deposited on cemented carbides [5, 8,9,10, 12, 13, 16, 18, 23], Si [6, 14, 15, 18], steel [4, 7, 11, 14, 20], and Ti alloy [19] using deposition techniques such as magnetron sputtering [6, 7, 11, 12, 14], arc ion plating [15, 18,19,20, 24], and cathodic arc deposition [4, 5, 8, 10, 13, 15, 16]. The synthesized microstructures are columnar [7, 11, 23], nanocomposites [6, 8, 14, 17, 21, 24], and multilayers [4, 5, 18]. Nanocomposite and multilayer microstructures attract special attention because of their unique merits, including superior mechanical, cutting, and lubricating properties [5, 18, 24], together with enhanced oxidation resistance owing to grain refinement and increment of Al-diffusion, which promoted preferential formation of the Al2O3 layer [16]. In contrast, the columnar microstructure exhibited poor oxidation resistance owing to fast inward O-diffusion and outward Ti-diffusion along grain boundaries [21]. TiAlSiN films have been comprised primarily of (TiN + AlN) [7], TiAlN [6, 7], (TiAlN + AlN) [12, 15], (TiAlN + TiN) [8], (TiN + AlSiN) [4, 22], and nanocomposites consisting of nanocrystalline (nc-) Ti(Al)N grains surrounded by an amorphous (a-) Si3N4 matrix [6, 11, 13, 14, 17, 21]. As such, Si existed either as a solid solution or a-Si3N4. Silicon effectively increased the hardness, cutting performance, thermal stability [11, 14], and oxidation resistance by forming a SiO2 barrier layer on TiAlSiN films [16]. It also refined grains and transformed the columnar microstructure to nanocomposites [12, 16].
Generally, oxidation of TiAlSiN films results in formation of α-Al2O3 and (rutile and/or anatase)-TiO2, with and without amorphous SiO2 in the temperature range of 500–1200 °C in air. The oxidized scales consisted of (TiO2, Al2O3)-mixture [19], (TiO2, Al2O3, SiO2)-mixture [17], (Al2O3-rich top layer)/(TiO2-rich sublayer) [5], (TiO2-rich top layer)/(Al2O3-rich sublayer) [15] with (TiO2, SiO2)-rich bottom layer [20], and nc-(TiO2, Al2O3) embedded in a-SiO2 matrix [21]. Although the high-temperature oxidation behavior of TiAlSiN films has been extensively studied, high-temperature corrosion in much more serious H2S–gas has not. TiAlSiN films could display good corrosion resistance because of their protective Al and Si elements.
In this study, multilayered TiAlSiN films were deposited by arc ion plating and corroded at high temperatures in an H2S-environment to investigate their corrosion behavior. Sulfur in fossil fuels, fluxes, and chemical feedstock is converted to H2S(g) during processing in reducing atmospheres of many modern technologies such as fossil fuel-fired power plants, coal gasification, petrochemical units, and petroleum refineries [25,26,27,28]. H2S-corrosion is serious, complex, and still not satisfactorily understood compared with oxidative attack. H2S dissociates into sulfur and hydrogen. Sulfur forms sulfides, which grow much faster than corresponding oxides owing to high concentration and/or mobility of point defects [25, 29]. Hydrogen atoms dissolve to cause embrittlement, migrate into the scale and metals interstitially, and generate point defects and hydrogen clusters, decreasing the corrosion resistance significantly [30]. This study analyzes the corrosion rates, formed scales, and corrosion mechanism of multilayered TiAlSiN film in harsh H2S environments at high temperatures.
2 Experimental Procedure
The TiAlSiN film was deposited on a precleaned 11 mm × 6 mm × 1.2 mm SUS304 (Fe–19%Cr–9%Ni) stainless steel plate to ~ 4 μm thickness by arc ion plating using Ti, Ti67Al33, and Al82Si18 cathodes under 0.005 Pa N2(g) at 450 °C, 100 A arc current, and − 50 V bias voltage. The film composition was 26Ti–16.3Al–1.2Si–56.5N (at%) according to electron probe microanalysis (EPMA). By rotating the steel substrate at 6 rpm among three opposing cathodes, the multilayered film was deposited.
The TiAlSiN film was corroded at 800, 900, and 1000 °C for 50 h using a thermogravimetric analyzer (TGA). Each sample was suspended by a quartz wire, and the weight change was continuously monitored as a function of corrosion time. During heating to the test temperature at a rate of 15 °C/min, Ar(g) was blown into the reaction chamber in TGA to avoid corrosion. At the test temperature, 1 atm of N2/0.1%H2S gas was blown into the reaction chamber to simulate the gas composition encountered in industrial coal gasification processing [26, 31]. The purity of N2 and H2S gas was 99.999% and 99.9%, respectively. No ultra-high-purity H2S gas was commercially available. Corroded samples were inspected using a field-emission EPMA, a field-emission scanning electron microscope (SEM), an Auger electron spectrometer (AES), a high-power X-ray diffractometer (XRD) with Cu-Kα radiation at 40 kV and 150 mA, and a transmission electron microscope (TEM operated at 200 kV) equipped with EDS (5 nm spot size). TEM samples were prepared by milling using a focused ion beam system after carbon coating. The concentrations of oxygen, nitrogen, and hydrogen in the corroded sample were measured in triplicate using the Leco ONH836 O/N/H analyzer, and the average values of the three measurements were used.
3 Results and Discussion
Figure 1 shows the SEM/TEM/EDS/XRD results of the TiAlSiN film deposited on the steel substrate. The film was dense, adherent, and about 4 μm thick (Fig. 1a). Its columnar microstructure consisted of a few nanometer-thick, alternating TiN/AlN layers parallel to the substrate surface (Fig. 1b). AlN nanolayers are brighter than TiN nanolayers because Al has a lower scattering factor than Ti (Fig. 1c). Though the EDS line profiles shown in Fig. 1d indicate alternating TiN and AlN nanolayers dissolved with (Al, Si) and (Ti, Si), respectively, they are inaccurate owing to the spurious Si signal produced by internal fluorescence of the EDS detector, overlapping of N-Kα and Ti-LIII spectra at 0.39 keV, and difficulty in quantifying lightweight elements such as nitrogen. Nonetheless, Debye rings superimposed with dots show the nanocrystalline film structure (Fig. 1e). Figure 1f indicates fcc-TiN and the austenitic stainless steel substrate. TiN peaks were broad due to the nanocrystalline film structure. hcp-AlN was not detected, because its amount was smaller than that of fcc-TiN [18].
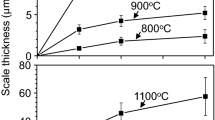
Figure 2 shows corrosion kinetics of the TiAlSiN film at 800–1000 °C. Total weight gains are relatively small, implying that the film had good corrosion resistance. With increasing temperature, weight gain increased owing to increased diffusion and reaction rates. The corrosion kinetics did not strictly follow the parabolic rate law due to the initial, transient corrosion stage; competitive corrosion of Al, Ti, and Si; evolution of nitrogen during scaling; and minute, local-scale spallation that occurred particularly at the later stage of corrosion.
After excluding the initial 5-h transient corrosion period, the parabolic rate constant, kp, was obtained from Fig. 2 using the equation (∆W/A)2 = kp·t, where ∆W/A is the weight gain per surface area of the specimen, and t is the corrosion time. In Fig. 3, kp values of the TiAlSiN film were compared with other published data [29, 30, 32,33,34]. They can be affected by oxidation/sulfidation/hydration rates of film-constituting elements and nitrogen escape. It is noted that Cr, Si [29], β-NiAl [32], and Ti [33, 35] oxidize to Cr2O3, SiO2, α-Al2O3, and TiO2, respectively, while Cr [34] and β-NiAl [30] sulfidize to Cr2S3 and NiAl2S4, respectively. Sulfidation rates are much faster than the corresponding oxidation rates, as shown in Fig. 3 [25, 29]. Values of kp for the TiAlSiN film were largely comparable to that of SiO2-forming kinetics, indicating that the film had good corrosion resistance even in harsh N2/0.1%H2S gas at high temperatures. This occurred because the corrosion was primarily governed not by sulfidation/hydration but oxidation, as will be explained below. The source for oxidation was impurity oxygen in N2/0.1%H2S gas.
Thermodynamic driving force for oxidation and sulfidation of the TiAlSiN film is shown in Fig. 4 [36]. The stability increased in the order of SiS2, Al2S3, TiS, SiO2, rutile-TiO2, TiO, and α-Al2O3. As such, oxides were more stable than the corresponding sulfides so that the film oxidized preferentially from the initial stage of corrosion (Fig. 5). The most stable α-Al2O3 grows very slowly because of its high stoichiometry. Preferential oxidation of Al is the main reason for the good corrosion resistance of the film (Figs. 5, 6, 7, 8). Titanium, whose oxide is slightly less stable than that of Al, oxidizes to stable rutile-TiO2 through transient oxides such as TiO, Ti2O3, and Ti4O7 [37]. Silicon, whose oxide is less stable than that of Ti, did not form SiO2 probably due to its small amount but dissolved in rutile-TiO2 and α-Al2O3, as shown in Fig. 9c. No sulfides were found in XRD analysis of the corroded film. Sulfur primarily dissolved in TiO2 and α-Al2O3 without forming sulfides, as shown in Fig. 9c.
Ellingham diagram of oxides and sulfides that can form on TiAlSiN film in N2/0.1%H2S gas [36]
Figure 5 shows the AES depth profiles of the corroded TiAlSiN film at the initial stage. To understand the corrosion mechanism, gold was sputter-deposited on the film prior to corrosion. The maximum concentration point of gold corresponds to the original film surface. Aluminum diffused outward to form the superficial Al2O3 oxide layer, where small amounts of Ti and sulfur dissolved. This indicates outward Ti-diffusion and inward S-diffusion across the superficial Al2O3 oxide layer. Also, oxygen diffused into the film, while nitrogen diffused out from the film, which was also observed during oxidation of TiAlSiN films [38, 39].
Figure 6 shows XRD/TEM/EDS results of the scale formed on the TiAlSiN film at the early corrosion stage. Weak patterns of Ti4O7, rutile-TiO2, and α-Al2O3 and strong patterns of steel substrate and film-constituting fcc-TiN were detected (Fig. 6a). Unlike sharp substrate peaks, TiN and all the oxide peaks were broad because of the nanocrystalline structure. Figure 6b shows 75 nm-thick scale consisting of Ti4O7, TiO2, and α-Al2O3 crystallites; 185 nm-thick oxygen affected film exhibiting AlN/TiN multilayers; and the uncorroded film consisting of weak, columnar, AlN/TiN multilayers. The oxide scale shown in Fig. 6b was divided into outer and inner layers as shown in Fig. 6c. Outer oxides were still fine but coarser than inner oxides due to growth at the free surface. Protruded outer oxides verified outward diffusion of Ti and Al ions (Fig. 6d). Inner oxides grew by inward diffusion of oxygen, which was substantiated by the presence of retained AlN/TiN multilayers. The fine, compact oxide scale shown in Fig. 6c could improve the corrosion resistance by hindering inward diffusion of oxygen and sulfur. The TEM–EDS spot analysis indicated that the average composition of the oxygen affected film was 0.7Ti–8.2Al–2.9Si–62.5N–25.7O–0.02S (at%), and that the oxide scale shown in Fig. 6b contained (0–2.4) at%S. Although the TEM-EDS analyzed compositions were inaccurate, it was seen that the oxygen affected film was dissolved with a large amount of oxygen, while the oxide scale contained very little sulfur.
When the TiAlSiN film was corroded further at a higher temperature, the intensity of diffraction decreased in the order of steel substrate, fcc-TiN, rutile-TiO2, and α-Al2O3 (Fig. 7a). Oxide peaks were still weak, exhibiting good corrosion resistance. No sulfides were detected owing to preferential oxidation of Ti and Al to rutile-TiO2 and α-Al2O3, respectively. About 0.5 μm-thick outer scale (o.s.), 0.2 μm-thick inner scale (i.s.), and 0.3 μm-thick oxygen affected film (O.A.F.) formed on the uncorroded film (Fig. 7b, c). The Ti–Al–O maps shown in Fig. 7c indicate that the upper part of the outer scale was rich in Al2O3, some of which might have been pre-formed during the initial/early corrosion period (Figs. 5, 6d). Formation of such Al2O3-rich scale facilitated formation of TiO2-rich scale in the lower part of the outer scale and in the inner scale (Fig. 7c). The grains of Al2O3 and TiO2 shown in Fig. 7b were submicrometer in size, suggesting that corrosion proceeded to a small extent. The good corrosion resistance of the TiAlSiN film is attributed to the follow. Firstly, nanocrystalline film oxidizes to tiny, dense, protective Al2O3 and semi-protective TiO2 crystallites from the initial corrosion stage. Secondly, Ti–Si bonds are stronger than Ti–Ti bonds, as indicated by the presence of five titanium silicides with high melting points in the Ti–Si binary phase diagram. Strong Ti–Si bonding hinders diffusion of less protective Ti and requires a higher activation energy for oxidation, leading to hindrance of TiO2-formation and thereby enhancement of Al2O3-formation from the initial corrosion stage. As such, the highly protective alumina scale can be more easily established in TiAlSiN film compared to Si-free TiAlN film. Thirdly, AlN is largely covalently bonded, while TiN has metallic bonding [40]. Such bonding suppresses diffusion of Al and Ti, leading to formation of refined, dense Al2O3 and TiO2 grains. Fourthly, the average composition of the oxygen affected TiAlSiN film was 1Ti–10.7Al–1.4Si–79.58N–7.3O–0.02S in at%, according to TEM/EDS spot analysis. Such a concentration was certainly erroneous, however, the formed oxides apparently deterred the ingress of sulfur.
Figure 8 shows XRD/EPMA results of the TiAlSiN film after corrosion at 900 °C for 100 h. Comparing Fig. 8a to Fig. 7a shows weaker diffraction patterns of fcc-TiN and substrate but stronger patterns of rutile-TiO2 and α-Al2O3 owing to increased corrosion time. Despite lengthy corrosion at a high temperature, a 2.3 μm-thick, uncorroded film was retained, over which 4 μm-thick, dense oxide scale was formed (Fig. 8b). The oxide scale was divided into three layers of 2 μm-thick, outer TiO2 layer; 1 μm-thick, intermediate, Al2O3-rich, TiO2-depleted layer; and 1 μm-thick, inner, Si-dissolved, TiO2-rich, Al2O3-depleted layer (Fig. 8c). Here, the outer TiO2 layer was formed by outward diffusion of Ti across the intermediate layer. Nonstoichiometric rutile-TiO2 grew much faster than stoichiometric α-Al2O3. The origin of the intermediate Al2O3-rich, TiO2-depleted layer shown in Fig. 8c might be the Al2O3-rich layer at the upper part of the outer scale shown in the Al map of Fig. 7c. Since Si is thermodynamically more noble than Ti or Al, it did not readily oxidize but accumulated in the inner oxide layer as dissolved ions (Fig. 8a, c). Under the current serious corrosion condition, the dense oxide scale shown in Fig. 8b could not prevent inward diffusion of oxygen and sulfur. Hence, sulfur was detected sporadically, as shown in the sulfur map of Fig. 8c. However, no sulfides were detected in Fig. 8a, due to their small amount or, more likely, dissolution of sulfur in oxides. Dissolution of Si and S in Al2O3 and TiO2 are illustrated in Fig. 9c. The concentrations of oxygen, nitrogen, and hydrogen in the sample depicted in Fig. 8 were measured using an O/N/H analyzer. The sample consisted of oxide scale, film, and substrate (Fig. 8b) and showed O/N/H concentrations of 0.2/0.17/0.0007 in wt%, representing a O/N/H ratio of 1/0.85/0.00035. There was very little hydrogen in the sample due probably to its limited solubility and diffusivity in oxides, although sulfur released from H2S was found sporadically over the oxide scale and retained film. Sulfur diffuses across the oxide scale by chemical diffusion as atoms and ions or physical permeation through voids, grain boundaries, dislocation pipes, or microcracks as S-bearing molecules [29].
The TiAlSiN film was destroyed when corroded for 50 h at 1000 °C, the maximum test temperature in this study. The thick scale was prone to spallation owing to corrosion by the (H, S, O)-mixed gas, growth stress produced by anisotropic volume expansion of oxides during scaling, and thermal stress arisen by mismatch in thermal expansion coefficients among oxides during cooling (Fig. 9a). Stress favored formation of numerous short whiskers on the scale surface (Fig. 9a). Whiskers at the surface and Kirkendall voids at the bottom of the spalled scale verified outward diffusion of Ti and Al during corrosion. Loosely adherent scales were partially embedded in the epoxy mount (Fig. 9b). Whiskers consisted of TiO2 and some Al2O3 (Fig. 9c). In those oxides, small amounts of sulfur and Si dissolved. Grains of TiO2 and Al2O3 near the surface were coarse owing to the highly corrosive condition (Fig. 9b). Figure 9c indicates that Si accumulated owing to its thermodynamic nobility in the lower part of the completely corroded film. In this area, Ti was depleted through its diffusion toward the surface, and Al was thereby enriched. Most of the sulfur was weakly detected in the upper part of the oxide scale.
The corrosion sequence of the TiAlSiN film at 800–1000 °C for 4–100 h in N2/0.1%H2S gas is schematically depicted in Fig. 10. At initial stage I, Al oxidized preferentially to form a superficial Al2O3 layer, according to thermodynamic activity and diffusivity (Fig. 5). From the initial stage, Al, Ti, and nitrogen diffused outward, while oxygen and a much smaller amount of sulfur diffused inward. At early stage II, in addition to Al, Ti diffused outward to form the outer (Al2O3, TiO2)-mixed scale, while inwardly diffused oxygen formed the inner (TiO2, Al2O3)-mixed scale, below which formed the oxygen affected film (Fig. 6). The superficial Al2O3 layer that formed at stage I was neither dense nor continuous enough to prevent oxidation of Ti. At early stage II, the outer oxide scale grew by outward (Ti, Al)-diffusion, whereas the inner oxide scale and the oxygen affected film grew by inward diffusion of oxygen and a much smaller amount of sulfur. The above counter-diffusion of Ti, Al, N, O, and S could occur for the following reasons: Firstly, α-Al2O3 grows by inward diffusion of O2− ions and weak outward diffusion of Al3+ ions [29]. Secondly, TiO2 grows by either outward diffusion of interstitial Ti4+ ions or inward diffusion of O2− ions, depending on defect concentration [41]. At intermediate stage III, counter-diffusion increased, and the bi-layered oxide scale and oxygen affected film increased in thickness. The outer oxide scale developed into the outer Al2O3-rich layer and the inner TiO2-rich layer, because Al oxidized more than Ti at the surface from the initial stage (Fig. 7). At late stage IV, nonstoichiometric TiO2 overgrew highly stoichiometric Al2O3, forming the outer TiO2-rich layer and the inner Al2O3-rich layer (Fig. 8). The outer TiO2-rich scale that formed by outward diffusion of Ti was thicker than the inner Al2O3-rich layer that formed by inward diffusion of oxygen. In the TiAlSiN film, Si was less active than Ti or Al so tended to be expelled from growing TiO2 and Al2O3, accumulating as dissolved ions in TiO2 and Al2O3 in the lower part of the oxide scale. At stage IV, the oxygen affected film was gone, because there was an enough amount of oxygen available. At final corrosion stage V, the film corroded completely (Fig. 9). The outer and inner scales were rich in TiO2 and Al2O3, respectively. Until film failure, the preferential reaction of Ti and Al with impurity oxygen in N2/0.1%H2S gas suppressed ingress of sulfur and hydrogen, effectively suppressing sulfidation and hydrogen attack.
4 Conclusions
The TiAlSiN multilayered film was deposited on steel substrate by arc ion plating. It consisted of a few nanometer-thick, alternating TiN/AlN nanocrystalline layers, in which Si was weakly distributed. The film was corroded at 800–1000 °C for 4–100 h in N2/0.1%H2S gas. During corrosion, Ti, Al, and nitrogen diffused outward, while oxygen and a smaller amount of sulfur diffused inward. Corrosion was mainly governed by oxidation, because oxides were thermodynamically more stable than corresponding sulfides. Impurity oxygen in N2/0.1%H2S gas preferentially reacted with the film to form (Ti, Al)-oxides, in which a small amount of Si dissolved. A superficial Al2O3 layer initially formed and then was overtaken by faster growing TiO2, leading to formation of outer TiO2-rich and inner Al2O3-rich oxide layers, below which the oxygen affected zone formed. Silicon tended to accumulate in the lower part of the oxide scale as dissolved ions owing to its thermodynamic nobility. TiAlSiN film exhibited good corrosion resistance for three main reasons. (1) Nanocrystalline film oxidized to dense Al2O3 and TiO2. (2) Strong Ti–Si, Al–N, and Ti–N bonding slowed the diffusion and reaction rates, hindering fast growth of oxide scales. (3) Preferential oxidation of Ti and furthermore Al led to formation of fine, dense TiO2 and Al2O3 grains, which suppressed ingress of sulfur and hydrogen.
References
H. Ichimura, A. Kawana, J. Mater. Res. 8, 1093 (1993)
S.H. Yao, Y.L. Su, W.H. Kao, T.H. Liu, Tribol. Int. 39, 332 (2006)
W.J. Cho, I.J. Shon, Korean J. Met. Mater. 56, 658 (2018)
S.K. Kim, V. Van Le, Thin Solid Films 518, 7483 (2010)
F. Pei, H.J. Liu, L. Chen, Y.X. Xu, Y. Du, J. Alloys Compd. 790, 909 (2019)
H. Chen, B.C. Zheng, Y.G. Li, Z.L. Wu, M.K. Lei, Thin Solid Films 669, 377 (2019)
F. Cao, P. Munroe, Z. Zhou, Z. Xie, Thin Solid Films 639, 137 (2017)
O. Nakonechna, T. Cselle, M. Morstein, A. Karimi, Thin Solid Films 447, 406 (2004)
L. Chen, S.Q. Wang, Y. Du, S.Z. Zhou, T. Gang, J.C. Fen, K.K. Chang, Y.W. Li, X. Xiong, Surf. Coat. Technol. 205, 582 (2010)
Y.Y. Chang, H.M. Lai, Surf. Coat. Technol. 259, 152 (2014)
W. Tillmann, M. Dildrop, Surf. Coat. Technol. 321, 448 (2017)
X. Sui, G. Li, X. Qin, H. Yu, X. Zhou, K. Wang, Q. Wang, Ceram. Int. 42, 7524 (2016)
C.L. Chang, J.W. Lee, M.D. Tseng, Thin Solid Films 517, 5231 (2009)
H.C. Barshilia, M. Ghosh, Shashidhara, R. Ramakrishna, K.S. Rajam, Appl. Surf. Sci. 256, 6420 (2010)
Y.Y. Chang, S.M. Yang, Thin Solid Films 518, s34 (2010)
L. Zhu, M. Hu, W. Ni, Y. Liu, Vacuum 86, 1795 (2012)
Z.W. Xie, L.P. Wang, X.F. Wang, L. Huang, Y. Lu, J.C. Yan, Nucl. Instrum. Methods B 271, 1 (2012)
S. Zhang, F. Cai, M. Li, Surf. Coat. Technol. 206, 3572 (2012)
L. Xin, Q. Chen, Y. Teng, W. Wang, A. Sun, S. Zhu, F. Wang, Surf. Coat. Technol. 228, 48 (2013)
C. Feng, M. Li, L. Xin, S. Zhu, Z. Shao, Q. Zhao, F. Wang, Surf. Coat. Technol. 232, 88 (2013)
T. Chen, Z. Xie, F. Gong, Z. Luo, Z. Yang, Appl. Surf. Sci. 314, 735 (2014)
S.H. Bak, D.B. Lee, Oxid. Met. 84, 345 (2015)
M. Parlinska-Wojtan, Thin Solid Films 616, 437 (2016)
J.H. Lee, H.K. Park, J.H. Jang, I.H. Oh, Met. Mater. Int. 25, 268 (2019)
G.Y. Lai, High-Temperature Corrosion and Materials Applications (ASM International, Cleveland, 1990), pp. 201–234
N.J. Simms, J.F. Norton, T.M. Lowe, J. Phys. IV 3, 807 (1993)
R. John, in Shreir’s Corrosion, vol. 1, 4th edn., ed. by R.A. Cottis, M.J. Graham, R. Lindsay, S.B. Lyon, J.A. Richardson, J.D. Scantlebury, F.H. Stott (Elsevier, London, 2010), pp. 240–271
Y. Feng, J. Wen, Y. Hu, B. Wu, M. Wua, J. Mi, Chem. Eng. J. 326, 1255 (2017)
N. Birks, G.H. Meier, F.S. Pettit, Introduction to the High-Temperature Oxidation of Metals, 2nd edn., (Cambridge University Press, Cambridge, 2006), pp. 195–197. 62, 63–204
E. Godlewska, Mater. Corros. 48, 687 (1997)
M.G. Hebsur, Appl. Energy 15, 99 (1983)
M.W. Brumm, H.J. Grabke, Corros. Sci. 33, 1677 (1992)
P. Kofstad, J. Less-Common Met. 12, 449 (1967)
S. Mrowec, M. Zastawnik, J. Phys. Chem. Solids 27, 1027 (1966)
Y.J. Kim, P. Yadav, J. Hahn, X. Xiao, D.B. Lee, Met. Mater. Int. 25, 627 (2019)
I. Barin, Thermochemical Data of Pure Substances (VCH, Germany, 1989)
A. Rahmel, P.J. Spencer, Oxid. Met. 35, 53 (1991)
A. Vennemann, H.R. Stock, J. Kohlscheen, S. Rambadt, G. Erkens, Surf. Coat. Technol. 174, 408 (2003)
M. Pfeiler, J. Zechner, M. Penoy, C. Michotte, C. Mitterer, M. Kathrein, Surf. Coat. Technol. 203, 3104 (2009)
H. Holleck, V. Schier, Surf. Coat. Technol. 76, 328 (1995)
Y.M. Chiang, D.P. Birnie III, W.D. Kingery, Physical Ceramics (Wiley, New York, 1996), p. 109
Acknowledgements
This work was supported by the National Research Council of Science and Technology (NST) Grant by the Korea government (MSIT) (No. CRC-15-07-KIER).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hahn, J., Abro, M.A., Xiao, X. et al. Corrosion of Multilayered TiAlSiN Films at 800–1000 °C in N2/0.1%H2S Gas. Met. Mater. Int. 27, 3260–3268 (2021). https://doi.org/10.1007/s12540-020-00665-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-020-00665-1