Abstract
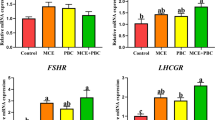
Previous study showed that dietary Bacillus licheniformis (B. licheniformis) administration contributes to the improvement of laying performance and egg quality in laying hens. In this study, we aimed to further evaluate its underlying mechanisms. Three hundred sixty Hy-Line Variety W-36 hens (28 weeks of age) were randomized into four groups, each group with six replications (n = 15). The control group received the basal diet and the treatment groups received the same basal diets supplemented with 0.01, 0.03, and 0.06% B. licheniformis powder (2 × 1010 cfu/g) for an 8-week trial. The results demonstrate that B. licheniformis significantly enhance the intestinal barrier functions via decreasing gut permeability, promoting mucin-2 transcription, and regulating inflammatory cytokines. The systemic immunity of layers in B. licheniformis treatment groups is improved through modulating the specific and non-specific immunity. In addition, gene expressions of hormone receptors, including estrogen receptor α, estrogen receptor β, and follicle-stimulating hormone receptor, are also regulated by B. licheniformis. Meanwhile, compared with the control, B. licheniformis significantly increase gonadotropin-releasing hormone level, but markedly reduce ghrelin and inhibin secretions. Overall, our data suggest that dietary inclusion of B. licheniformis can improve the intestinal barrier function and systemic immunity and regulate reproductive hormone secretions, which contribute to better laying performance and egg quality of hens.



Similar content being viewed by others
References
Khan SH, Atif M, Mukhtar N, Rehman A, Fareed G (2011) Effects of supplementation of multi-enzyme and multi-species probiotic on production performance, egg quality, cholesterol level and immune systemic in laying hens. J Appl Anim Res 39:386–398
Nahashon SN, Nakaue HS, Mirosh LW (1994a) Production variables and nutrient retention in single comb White Leghorn laying pullets fed diets supplemented with direct-fed microbials. Poult Sci 73:1699–1711
Nahashon SN, Nakaue HS, Snyder SP, Mirosh LW (1994b) Performance of single comb White Leghorn layers fed corn-soybean meal and barley-corn-soybean meal diets supplemented with a direct-fed microbial. Poult Sci 73:1712–1723
Jin LZ, Ho YW, Abdullah N, Jalaludin S (1997) Probiotics in poultry: modes of action. World Poultry Sci J 53:351–368
Coates ME, Fuller R (1977) The genotobiotic animal in the study of gut microbiology. Microbial ecology of the gut. Academic Press, London, pp 311–346
Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T (2000) Probiotic bacteria: safety, functional and technological properties. J Biotechnol 84:197–215
Salianeha N, Shirzadb MR, Seifi S (2011) Performance and antibody response of broiler chickens fed diets containing probiotic and prebiotic. J Appl Anim Res 39:65–67
Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130(2S Suppl):396S–402S
Tannock GW (2001) Molecular assessment of intestinal microflora. Am J Clin Nutr 73:410S–414S
Hong HA, Duc LH, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835
Hagedorn SR, Bradley G, Chapman PJ (1985) Glutathione-independent isomerization of maleylpyruvate by Bacillus megaterium and other gram-positive bacteria. J Bacteriol 163:640–647
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28:214–220
Liu X, Yan H, Lv L, Xu Q, Yin C, Zhang K, Wang P, Hu J (2012) Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas J Anim Sci 25:682–689
Rozs M, Manczinger L, Vagvolgyi C, Kevei F (2001) Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiol Lett 205:221–224
Kim Y, Cho JY, Kuk JH, Moon JH, Cho JI, Kim YC, Park KH (2004) Identification and antimicrobial activity of phenylacetic acid produced by Bacillus licheniformis isolated from fermented soybean, Chungkook-Jang. Curr Microbiol 48:312–317
Lei K, Li YL, Yu DY, Rajput IR, Li WF (2013) Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult Sci 92:2389–2395
Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC (2009) Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15:300–310
Nikoskelainen S, Ouwehand AC, Bylund G, Salminen S, Lilius E (2003) Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish Shellfish Immunol 15:443–452
Zuberbuehler CA, Messikommer RE, Arnold MM, Forrer RS, Wenk C (2006) Effects of selenium depletion and selenium repletion by choice feeding on selenium status of young and old laying hens. Physiol Behav 87:430–440
Cui X, Li Y, Liu R, Zheng M, Li Q, Wen J (2016) Follicle-stimulating hormone increases the intramuscular fat content and expression of lipid biosynthesis genes in chicken breast muscle. J Zhejiang Univ Sci B 17:303
Lei K, Li YL, Wang Y, Wen J, Wu HZ, Yu DY, Li WF (2015) Effect of dietary supplementation of Bacillus subtilis B10 on biochemical and molecular parameters in the serum and liver of high-fat diet-induced obese mice. J Zhejiang Univ Sci B 16:487–495
Hata H, Sakaguchi N, Yoshitomi H et al (2004) Distinct contribution of IL-6, TNF-α, IL-1, and IL-10 to T cell–mediated spontaneous autoimmune arthritis in mice. J Clin Invest 114:582–588
Anderson JM, Van Italie CM, Fanning AS (2004) Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 16:140–145
Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153
Deng W, Dong XF, Tong JM, Zhang Q (2012) The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult Sci 91:575–582
Yousefi M, Karkoodi K (2007) Effect of probiotic Thepax® and Saccharomyces cerevisiae supplementation on performance and egg quality of laying hens. Int J Poult Sci 6:52–54
Yörük MA, Gül M, Hayirli A, Macit M (2004) The effects of supplementation of humate and probiotic on egg production and quality parameters during the late laying period in hens. Poult Sci 83:84–88
Zihni C, Balda MS, Matter K (2014) Signaling at tight junctions during epithelial differentiation and microbial pathogenesis. J Cell Sci 127:3401–3413
Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M (2009) Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 296:G1140–G1149
Ukena SN, Singh A, Dringenberg U et al (2007) Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One 2:e1308
Resta-Lenert S, Barrett KE (2003) Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52:988–997
Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105:15064–15069
Caballero-Franco C, Keller K, De Simone C, Chadee K (2007) The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 292:G315–G322
Shimazu T, Villena J, Tohno M, Fujie H, Hosoya S, Shimosato T et al (2012) Immunobiotic Lactobacillus jensenii elicits anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the toll-like receptor signaling pathway. Infect Immun 80:276–288
Perdigon G, Maldonado Galdeano C, Valdez JC, Medici M (2002) Interaction of lactic acid bacteria with the gut immune system. Eur J Clin Nutr 56(Suppl 4):S21–S26
Liu YL, Huang JJ, Hou YQ et al (2008) Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr 100:552–560
Huang Y, Li YL, Huang Q et al (2012) Effect of orally administered Enterococcus faecium EF1 on intestinal cytokines and chemokines production of suckling piglets. Pak Vet J 32:81–84
Al-Sadi R, Boivin M, Ma T (2009) Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci 14:2765–2778
Smit HF, Dwars RM, Davelaar FG, Wijtten GA (1998) Observations on the influence of intestinal spirochaetosis in broiler breeders on the performance of their progeny and on egg production. Avian pathol: journal of the WVPA 27:133–141
Izcue A, Coombes JL, Powrie F (2006) Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev 212:256–271
Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S (2001) Probiotics: effects on immunity. Am J Clin Nutr 73:444S–450S
Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME (2009) Probiotics and immunity. J Gastroenterol 44:26–46
Benyacoub J, Czarnecki-Maulden GL, Cavadini C, Sauthier T, Anderson RE, Schiffrin EJ, von der Weid T (2003) Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. J Nutr 133:1158–1162
Koenen ME, Kramer J, van der Hulst R, Heres L, Jeurissen SH, Boersma WJ (2004) Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. Br Poult Sci 45:355–366
Panda AK, Rama Rao SS, Raju VLN, Sharma SS (2008) Effect of probiotic (Lactobacillus sporogenes) feeding on egg production and quality, yolk cholesterol and humoral immune response of White Leghorn layer breeders. J Sci Food Agr 88:43–47
Asli MM, Hosseini SA, Lotfollahian H, Shariatmadari F (2007) Effect of probiotics, yeast, vitamin E and vitamin C supplements on performance and immune response of laying hen during high environmental temperature. IntJ Poultry Sci 6:895–900
Klasing KC (1988) Nutritional aspects of leukocytic cytokines. J Nutr 118:1436–1446
van Eck JH (1983) Effects of experimental infection of fowl with EDS’76 virus, infectious bronchitis virus and/or fowl adenovirus on laying performance. Vet Q 5:11–25
Hampson DJ, McLaren AJ (1999) Experimental infection of laying hens with Serpulina intermedia causes reduced egg production and increased faecal water content. Avian Pathol 28:113–117
Smith CA, Andrews JE, Sinclair AH (1997) Gonadal sex differentiation in chicken embryos: expression of estrogen receptor and aromatase genes. J Steroid Biochem Mol Biol 60:295–302
Zhao LH, Chen JL, Xu H, Liu JW, Xu RF (2010) Cloning and expression of FSHb gene and the effect of FSHβ on the mRNA levels of FSHR in the local chicken. Asian Austral J Anim 23:292–301
Wang XJ, Li Y, Song QQ, Guo YY, Jiao HC, Song ZG, Lin H (2013) Corticosterone regulation of ovarian follicular development is dependent on the energy status of laying hens. J Lipid Res 54:1860–1876
Ikemoto T, Park MK (2007) Comparative analysis of the pituitary and ovarian GnRH systemics in the leopard gecko: signaling crosstalk between multiple receptor subtypes in ovarian follicles. J Mol Endocrinol 38:289–304
Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S (2001) Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun 281:1220–1225
Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L (2004) Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett 362:103–107
Fernandez-Fernandez R, Tena-Sempere M, Roa J, Castellano JM, Navarro VM, Aguilar E, Pinilla L (2007) Direct stimulatory effect of ghrelin on pituitary release of LH through a nitric oxide-dependent mechanism that is modulated by estrogen. Reproduction 133:1223–1232
Jimenez-Krassel F, Winn ME, Burns D, Ireland JL, Ireland JJ (2003) Evidence for a negative intrafollicular role for inhibin in regulation of estradiol production by granulosa cells. Endocrinology 144:1876–1886
Matzuk MM, Finegold MJ, Su J-GJ, Hsueh AJW, Bradley A (1992) Inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360:313–319
Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A (1994) Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci U S A 91:8817–8821
Kumar TR, Wang Y, Matzuk MM (1996) Gonadotropins are essential modifier factors for gonadal tumor development in inhibin-deficient mice. Endocrinology 137:4210–4216
Acknowledgements
This study was supported by The National 863 Project (Grant No. 013AA102803D) and the Key Science and Technology Program of Zhejiang Province, China (Grant No. 2006C12086).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal manipulations were conducted in accordance with the guidelines for animal welfare and approved by the Animal Welfare Committee of Animal Science College, Zhejiang University.
Rights and permissions
About this article
Cite this article
Wang, Y., Du, W., Lei, K. et al. Effects of Dietary Bacillus licheniformis on Gut Physical Barrier, Immunity, and Reproductive Hormones of Laying Hens. Probiotics & Antimicro. Prot. 9, 292–299 (2017). https://doi.org/10.1007/s12602-017-9252-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9252-3