Abstract
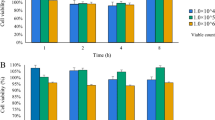
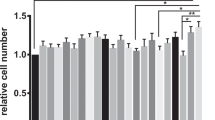
Probiotic as a preventive medicine is emerging as an indispensable tool in addressing the foodborne infections or gastrointestinal disorders. The present study was sought to determine the in vitro prophylactic potential of probiotic Lactobacillus rhamnosus (LR: MTCC-5897) against Escherichia coli (ATCC 14948) induced impairment in intestinal barrier function using Caco-2 cells. Intestinal cells exposed to E. coli demonstrated significantly higher phenol red flux (p < 0.05) and concomitantly decreased TEER (0.69 ± 0.01) in contrast to control or L. rhamnosus (109 cfu/mL)-treated cells. However, E. coli-induced barrier hyperpermeability was restored to significant extents (p < 0.01) when E. coli were excluded, competed or displaced by probiotic LR. Similarly, exposure of Caco-2 cells to E. coli reduced the mRNA expression of key tight junction genes, viz. Zo-1, Claudin-1, Occludin and Cingulin which however were restored significantly (p < 0.05) with L. rhamnosus treatment during exclusion or competition than displacement assays. The protective behaviour of probiotic LR against E. coli can also be observed in immunofluorescent and electron micrograph where intact cellular morphology along with preserved distribution and localisation of key integrity proteins can be found in LR-treated cells in contrast to distorted and disorganised distribution observed with E. coli exposure. In conclusion, L. rhamnosus inhibited and re-established E. coli-impaired intestinal barrier function by improving the expression and distribution of key junction protein and hence could serve an essential food additive to address the various health complications especially those associated with gastrointestinal tract.





Similar content being viewed by others
References
Laskowska E, Jarosz LS, Grądzki Z (2018) Effect of the EM Bokashi® multimicrobial probiotic preparation on the non-specific immune response in pigs. Probiotics Antimicrob Proteins:1–14. https://doi.org/10.1007/s12602-018-9460-5
Cerdo T, Ruiz A, Suarez A, Campoy C (2017) Probiotic, prebiotic and brain development. Nutrients 9:1247
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26:26191
Zielinska D, Dlugosz E, Zawistowska-Deniziak A (2018) Functional properties of food origin Lactobacillus in the gastrointestinal ecosystem in vitro Study. Probiotics Antimicrob Proteins 23:1–10
Dahiya DK, Puniya M, Shandilya UK, Dhewa T, Kumar N, Kumar S, Puniya AK, Shukla P (2017) Gut microbiota modulation and its relationship with obesity using prebiotic fibres and probiotics: a review. Front Microbiol 8:563
Garcia MA, Nelson WJ, Chavez N (2018) Cell–cell junctions organize structural and signaling networks. Cold Spring Harb Perspect 10:029181
Hammer AM, Morris NL, Earley ZM, Choudhry MA (2015) The first line of defense: the effects of alcohol on post-burn intestinal barrier, immune cells and microbiome. Alcohol Res 37:209
Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM (2014) Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol 14:189
Mao X, Gu C, Hu H, Tang J, Chen D, Yu B, He J, Yu J, Luo J, Tian G (2016) Dietary Lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by porcine rotavirus. PLoS One 11:e0146312
Secher T, Kassem S, Benamar M, Bernard I, Boury M, Barreau F, Oswald E, Saoudi A (2017) Oral administration of the probiotic strain Escherichia coli Nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front Immunol 8:1096
Ekmekciu I, von Klitzing E, Fiebiger U, Neumann C, Bacher P, Scheffold A, Bereswill S, Heimesaat MM (2017) The probiotic compound VSL# 3 modulates mucosal, peripheral and systemic immunity following murine broad-spectrum antibiotic treatment. Front Cell Infect Microbiol 7:167
Sharma R, Kapila R, Dass G, Kapila S (2014) Improvement in Th1/Th2 immune homeostasis, antioxidative status and resistance to pathogenic E. coli on consumption of probiotic Lactobacillus rhamnosus fermented milk in aging mice. Age 36:9686
Saliganti V, Kapila R, Kapila S (2016) Consumption of probiotic Lactobacillus rhamnosus (MTCC: 5897) containing fermented milk plays a key role in development of the immune system in newborn mice during the suckling–weaning transition. Microbiol Immunol 60:261–267
Saliganti V, Kapila R, Sharma R, Kapila S (2015) Feeding probiotic Lactobacillus rhamnosus (MTCC 5897) fermented milk to suckling mothers alleviates ovalbumin-induced allergic sensitisation in mice offspring. Br J Nutr 114:1168–1179
Bhat MI, Singh VK, Sharma D, Kapila S, Kapila R (2019) Adherence capability and safety assessment of an indigenous probiotic strain Lactobacillus rhamnosus MTCC-5897. Microb Pathog 130:120–130
Bhat MI, Kumari A, Kapila S, Kapila R (2019) Probiotic lactobacilli mediated changes in global epigenetic signatures of human intestinal epithelial cells during Escherichia coli challenge. Ann Microbiol 2019:1–10
Zargar A, Quan DN, Carter KK, Guo M, Sintim HO, Payne GF, Bentley WE (2015) Bacterial secretions of nonpathogenic Escherichia coli elicit inflammatory pathways: a closer investigation of interkingdom signaling. MBio 6:e00025–e00015
Nakata K, Sugi Y, Narabayashi H, Kobayakawa T, Nakanishi Y, Tsuda M, Hosono A, Kaminogawa S, Hanazawa S, Takahashi K (2017) Commensal microbiota-induced microRNA modulates intestinal epithelial permeability through the small GTPase ARF4. J Biol Chem 29:15426–15433
Briske-Anderson MJ, Finley JW, Newman SM (1997) The influence of culture time and passage number on the morphological and physiological development of Caco-2 cells. Proc Soc Exp Biol Med 214:248–257
Barnett A, Roy N, McNabb W, Cookson A (2016) Effect of a semi-purified oligosaccharide-enriched fraction from caprine milk on barrier integrity and mucin production of co-culture models of the small and large intestinal epithelium. Nutrients 8:267
Bhat MI, Sowmya K, Kapila S, Kapila R (2019) Escherichia coli K12: an evolving opportunistic commensal gut microbe distorts barrier integrity in human intestinal cells. Microb Pathog 133:103545
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods 25:402–408
Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, Turner JR (2001) Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na + -glucose cotransport. Am J Physiol Gastrointest Liver Physiol 28:1487–1493
Coconnier MH, Lievin V, Lorrot M, Servin AL (2000) Antagonistic activity of Lactobacillus acidophilus LB against intracellular Salmonella enterica serovar Typhimurium infecting human enterocyte-like Caco-2/TC-7 cells. Appl Environ Microbiol 66:1152–1157
West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, Prescott SL, MacKay C, Salminen S, Wong G, Sinn J (2015) The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol 135:3–13
Markowiak P, Slizewska K (2017) Effects of probiotics, prebiotics and synbiotics on human health. Nutrients 9:1021
Bambou JC, Giraud A, Menard S, Begue B, Rakotobe S, Heyman M, Taddei F, Cerf-Bensussan N, Gaboriau-Routhiau V (2004) In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J Biol Chem 279:42984–42992
Koli P, Sudan S, Fitzgerald D, Adhya S, Kar S (2011) Conversion of commensal Escherichia coli K-12 to an invasive form via expression of a mutant histone-like protein. MBio 2:e00182–e00111
Kim SC, Tonkonogy SL, Karrasch T, Jobin C, Sartor BR (2007) Dual-association of gnotobiotic IL-10−/− mice with 2 nonpathogenic commensal bacteria induces aggressive pancolitis. Inflamm. Bowel Dis 13:1457–1466
Loh G, Blaut M (2012) Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes 3:544–555
Ismail M (1999) The use of Caco-2 cells as an in vitro method to study bioavailability of iron. Malays J Nutr 5:31–45
Tan HY, Trier S, Rahbek UL, Dufva M, Kutter JP, Andresen TL (2018) A multi-chamber microfluidic intestinal barrier model using Caco-2 cells for drug transport studies. PLoS One 13:e0197101
Han C, Ding Z, Shi H, Qian W, Hou X, Lin R (2016) The role of probiotics in lipopolysaccharide-induced autophagy in intestinal epithelial cells. Cell Physiol Biochem 38:2464–2478
Ziegler AL, Pridgen TA, Mills JK, Gonzalez LM, Van Landeghem L, Odle J, Blikslager AT (2018) Epithelial restitution defect in neonatal jejunum is rescued by juvenile mucosal homogenate in a pig model of intestinal ischemic injury and repair. PLoS One 13:e0200674
Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM (2010) Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Phys Gastrointest Liver 298:851–859
Lodemann U, Strahlendorf J, Schierack P, Klingspor S, Aschenbach JR, Martens H (2015) Effects of the probiotic Enterococcus faecium and pathogenic Escherichia coli strains in a pig and human epithelial intestinal cell model. Scientifica 2015:235184
Samtiya M, Bhat MI, Gupta T, Kapila S, Kapila R (2019) Safety assessment of potential probiotic Lactobacillus fermentum MTCC-5898 in murine model after repetitive dose for 28 days (Sub-Acute Exposure). Probiotics Antimicrob Proteins 8:1–2
Llewellyn A, Foey A (2017) Probiotic modulation of innate cell pathogen sensing and signalling events. Nutrients 9:1156
Alvarez CS, Badia J, Bosch M, Gimenez R, Baldoma L (2016) Outer membrane vesicles and soluble factors released by probiotic Escherichia coli Nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front Microbiol 7:1981
Rhayat L, Maresca M, Nicoletti C, Perrier J, Brinch KS, Christian S, Devillard E, Eckhardt E (2019) Effect of Bacillus subtilis strains on intestinal barrier function and inflammatory response. Front Immunol 10:564
Lepine AF, de Wit N, Oosterink E, Wichers H, Mes J, de Vos P (2018) Lactobacillus acidophilus attenuates Salmonella-induced stress of epithelial cells by modulating tight-junction genes and cytokine responses. Front Microbiol 9:1439
Blackwood BP, Yuan CY, Wood DR, Nicolas JD, Grothaus JS, Hunter CJ (2017) Probiotic Lactobacillus species strengthen intestinal barrier function and tight junction integrity in experimental necrotizing enterocolitis. J Probiotics Health 5:159
Qiu Y, Jiang Z, Hu S, Wang L, Ma X, Yang X (2017) Lactobacillus plantarum enhanced IL-22 production in natural killer (NK) cells that protect the integrity of intestinal epithelial cell barrier damaged by enterotoxigenic Escherichia coli. Int J Mol Sci 18:2409
Moon C, VanDussen KL, Miyoshi H, Stappenbeck TS (2014) Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol 7:818
Buckley A, Turner JR (2018) Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol 10:029314
Johnson-Henry KC, Donato KA, Shen-Tu G, Gordanpour M, Sherman PM (2008) Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect Immun 76:1340–1348
Roselli M, Finamore A, Hynonen U, Palva A, Mengheri E (2016) Differential protection by cell wall components of Lactobacillus amylovorus DSM 16698 T against alterations of membrane barrier and NF-kB activation induced by enterotoxigenic F4+ Escherichia coli on intestinal cells. BMC Microbiol 16:226
Shi CZ, Chen HQ, Liang Y, Xia Y, Yang YZ, Yang J, Zhang JD, Wang SH, Liu J, Qin HL (2014) Combined probiotic bacteria promotes intestinal epithelial barrier function in interleukin-10-gene-deficient mice. World J Gastroenterol 20:4636
Acknowledgments
We are highly indebted to the Department of BioTechnology, Ministry of Science and Technology, New Delhi for providing necessary funds (BT/PR15109/PFN/20/1174/2015) required for the present work. The funding agency has no role in the design, analysis or writing of this article. The authors also acknowledge ICAR National Dairy Research Institute (NDRI) Karnal, for providing the laboratory facilities for execution of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhat, M.I., Sowmya, K., Kapila, S. et al. Potential Probiotic Lactobacillus rhamnosus (MTCC-5897) Inhibits Escherichia coli Impaired Intestinal Barrier Function by Modulating the Host Tight Junction Gene Response. Probiotics & Antimicro. Prot. 12, 1149–1160 (2020). https://doi.org/10.1007/s12602-019-09608-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-019-09608-8