Abstract
Grape pomace is an abundant winery by-product produced worldwide, which contains a high concentration of polyphenols trapped in cell wall fibers. The fungus tannase enzyme finds many applications in the industry, but its use is currently limited. This is due to its high production cost derived from tannic acid, which is the typical inductor of tannase enzyme by Aspergillus species. Therefore, assessment of natural tannin sources as inductors is a strategy to overcome this limitation. We propose here to employ the red grape pomace, which is a rich source of tannins and polyphenols. We found that, although grape pomace is not able to induce tannase by itself, it is a useful complement for tannic acid induction, reducing the concentration of tannic acid necessary to achieve maximum levels of tannase induction, which ranged between 3.0 and 4.5 U/mL. We also explored the potential usage of this biomass to induce other relevant industrial enzymes and quantified the recovery of gallic acid from grape pomace by the fungus fermentation; finding new routes for this by-product valorisation.
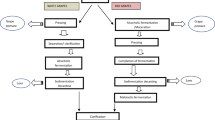
Graphic Abstract





Similar content being viewed by others
References
Zacharof, M.-P.: Grape winery waste as feedstock for bioconversions: applying the biorefinery concept. Waste Biomass Valor. 8, 1011–1025 (2017). https://doi.org/10.1007/s12649-016-9674-2
Fontana, A.R., Antoniolli, A., Bottini, R.: Grape pomace as a sustainable source of bioactive compounds: extraction, characterization, and biotechnological applications of phenolics. J Agric. Food Chem. 61, 8987–9003 (2013). https://doi.org/10.1021/jf402586f
Belur, P.D., Mugeraya, G.: Microbial production of tannase: state of the art. Res. J. Microbiol. 6, 25–40 (2011). https://doi.org/10.3923/jm.2011.25.40
Boadi, D.K., Neufeld, R.J.: Encapsulation of tannase for the hydrolysis of tea tannins. Enzyme Microb. Technol. 28, 590–595 (2001)
Lekha, P.K., Ramakrishna, M., Lonsane, B.K.: Strategies for the isolation of potent fungal cultures capable of producing tannin acyl hydrolase in higher titres. Chem. Mikrobiol. Technol. Lebensm. 15, 5–10 (1993)
Belmares, R., Contreras-Esquivel, J.C., Rodrı́guez-Herrera, R., Coronel, A.R., Aguilar, C.N.: Microbial production of tannase: an enzyme with potential use in food industry. LWT Food Sci. Technol. 37, 857–864 (2004). https://doi.org/10.1016/j.lwt.2004.04.002
Deschamps, A.M., Lebeault, J.-M.: Production of gallic acid from tara tannin by bacterial strains. Biotechnol. Lett. 6, 237–242 (1984). https://doi.org/10.1007/BF00140043
Murugan, K., Al-Sohaibani, S.A.: Biocompatible removal of tannin and associated color from tannery effluent using the biomass and tannin acyl hydrolase (E.C.3.1.1.20) enzymes of mango industry solid waste isolate Aspergillus candidus MTTC 9628. Res. J. Microbiol. 5, 262–271 (2010). https://doi.org/10.3923/jm.2010.262.271
Abdulla, J., Rose, S.P., Mackenzie, A.M., Mirza, W., Pirgozliev, V.: Exogenous tannase improves feeding value of a diet containing field beans (Vicia faba) when fed to broilers. Br. Poult. Sci. 57, 246–250 (2016). https://doi.org/10.1080/00071668.2016.1143551
Aboubakr, H.A., El-Sahn, M.A., El-Banna, A.A.: Some factors affecting tannase production by Aspergillus niger Van Tieghem. Braz. J. Microbiol. 44, 559–567 (2013). https://doi.org/10.1590/S1517-83822013000200036
Sabu, A., Pandey, A., Daud, M.J., Szakacs, G.: Tamarind seed powder and palm kernel cake: two novel agro residues for the production of tannase under solid state fermentation by Aspergillus niger ATCC 16620. Bioresour. Technol. 96, 1223–1228 (2005). https://doi.org/10.1016/j.biortech.2004.11.002
Treviño-Cueto, B., Luis, M., Contreras-Esquivel, J.C., Rodríguez, R., Aguilera, A., Aguilar, C.N.: Gallic acid and tannase accumulation during fungal solid state culture of a tannin-rich desert plant (Larrea tridentata Cov.). Bioresour. Technol. 98, 721–724 (2007). https://doi.org/10.1016/j.biortech.2006.02.015
Kumar, R., Sharma, J., Singh, R.: Production of tannase from Aspergillus ruber under solid-state fermentation using jamun (Syzygium cumini) leaves. Microbiol. Res. 162, 384–390 (2007). https://doi.org/10.1016/j.micres.2006.06.012
Sharma, N.K., Beniwal, V., Kumar, N., Kumar, S., Pathera, A.K., Ray, A.: Production of tannase under solid-state fermentation and its application in detannification of guava juice. Prep. Biochem. Biotechnol. 44, 281–290 (2014). https://doi.org/10.1080/10826068.2013.812566
Singleton, V.L., Rossi, J.A.: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965)
Hagerman, A.E., Butler, L.G.: Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 26, 809–812 (1978). https://doi.org/10.1021/jf60218a027
AOAC International: AOAC: Official methods of analysis (vol 1). https://archive.org/details/gov.law.aoac.methods.1.1990. (1990)
ANKOM Technology: Neutral detergent fiber in feeds—method 6 (2011)
ANKOM Technology: Acid detergent fiber in feeds—method 5 (2011)
ANKOM Technology: Acid detergent lignin—PROMEFA V2 protocol (2005)
Mangrola, A.V., Patel, H.V., Chudasama, C.J., Vavadia, C.N., Shah, H.: Optimization of cultural conditions for tannase production in submerged fermentation by Aspergillus niger avm-1. Pharm. Res. 8
Sharma, S., Bhat, T.K., Dawra, R.K.: A spectrophotometric method for assay of tannase using rhodanine. Anal. Biochem. 279, 85–89 (2000). https://doi.org/10.1006/abio.1999.4405
Smith, P.K., Krohn, R.I., Hermanson, G.T., Mallia, A.K., Gartner, F.H., Provenzano, M.D., Fujimoto, E.K., Goeke, N.M., Olson, B.J., Klenk, D.C.: Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85 (1985). https://doi.org/10.1016/0003-2697(85)90442-7
Brown, R.E., Jarvis, K.L., Hyland, K.J.: Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal. Biochem. 180, 136–139 (1989)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959). https://doi.org/10.1021/ac60147a030
Gupta, M.N., Dong, G., Mattiasson, B.: Purification of endo-polygalacturonase by affinity precipitation using alginate. Biotechnol. Appl. Biochem. 18(Pt 3), 321–327 (1993)
Ncube, T., Howard, R.L., Abotsi, E.K., van Rensburg, E.L.J., Ncube, I.: Jatropha curcas seed cake as substrate for production of xylanase and cellulase by Aspergillus niger FGSCA733 in solid-state fermentation. Ind. Crops Prod. 37, 118–123 (2012). https://doi.org/10.1016/j.indcrop.2011.11.024
Porfiri, M.C., Farruggia, B.M., Romanini, D.: Bioseparation of alpha-amylase by forming insoluble complexes with polyacrylate from a culture of Aspergillus oryzae grown in agricultural wastes. Sep. Purif. Technol. 92, 11–16 (2012). https://doi.org/10.1016/j.seppur.2012.03.004
Lekha, P.K., Lonsane, B.K.: Comparative titres, location and properties of tannin acyl hydrolase produced by Aspergillus niger PKL 104 in solid-state, liquid surface an submerged fermentations. Process Biochem. 29, 497–503 (1994). https://doi.org/10.1016/0032-9592(94)85019-4
Bradoo, S., Gupta, R., Saxena, R.K.: Parametric optimization and biochemical regulation of extracellular tannase from Aspergillus japonicus. Process Biochem. 32, 135–139 (1997)
Sharma, S., Agarwal, L., Saxena, R.K.: Statistical optimization for tannase production from Aspergillus niger under submerged fermentation. Indian J. Microbiol. 47, 132–138 (2007). https://doi.org/10.1007/s12088-007-0026-6
Darah, I., Sumathi, G., Jain, K., Hong, L.S.: Involvement of physical parameters in medium improvement for tannase production by Aspergillus niger FETL FT3 in submerged fermentation. Biotechnol. Res. Int. 2011, 897931 (2011)
Aissam, H., Errachidi, F., Penninckx, M.J., Merzouki, M., Benlemlih, M.: Production of tannase by Aspergillus niger HA37 growing on tannic acid and olive mill waste waters. World J. Microbiol. Biotechnol. 21, 609–614 (2005)
Aguilar, C.N., Augur, C., Favela-Torres, E., Viniegra-González, G.: Production of tannase by Aspergillus niger Aa-20 in submerged and solid-state fermentation: influence of glucose and tannic acid. J. Ind. Microbiol. Biotechnol. 26, 296–302 (2001)
Favela-Torres, E., Cordova-López, J., García-Rivero, M., Gutiérrez-Rojas, M.: Kinetics of growth of Aspergillus niger during submerged, agar surface and solid state fermentations. Process Biochem. 33, 103–107 (1998). https://doi.org/10.1016/S0032-9592(97)00032-0
Yadav, K.K., Garg, N., Kumar, D., Kumar, S., Singh, A., Muthukumar, M.: Application of response surface methodology for optimization of polygalacturonase production by Aspergillus niger. J. Environ. Biol. 36, 255–259 (2015)
Barman, S., Sit, N., Badwaik, L.S., Deka, S.C.: Pectinase production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J. Food Sci. Technol. 52, 3579–3589 (2015). https://doi.org/10.1007/s13197-014-1413-8
Dias, L.M., Dos Santos, B.V., Albuquerque, C.J.B., Baeta, B.E.L., Pasquini, D., Baffi, M.A.: Biomass sorghum as a novel substrate in solid-state fermentation for the production of hemicellulases and cellulases by Aspergillus niger and A. fumigatus. J. Appl. Microbiol. 124, 708–718 (2018). https://doi.org/10.1111/jam.13672
Acknowledgements
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PICT-2016-4463 and PICT-2016-1170). The authors would like to thank BordeRío Bodega & Viñedos, AER INTA-Victoria, and Bodegas Salentein, Argentina for supplying the grape pomace.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meini, MR., Ricardi, L.L. & Romanini, D. Novel Routes for Valorisation of Grape Pomace Through the Production of Bioactives by Aspergillus niger. Waste Biomass Valor 11, 6047–6055 (2020). https://doi.org/10.1007/s12649-019-00844-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00844-1