Abstract
Aluminium dross, an industrial by-product generated in large quantities, is fairly rich in aluminium content. Even though there are means of recycling (pyrometallurgical and hydrometallurgical routes), still a large amount of aluminium dross is landfilled. This research article emphasizes on recycling of aluminium dross by the production of potash alum. Powdered aluminium dross is allowed to react with potassium hydroxide, followed in sequence by filtration, slow addition of sulphuric acid coupled with constant stirring of the solution, heating the solution and cooling it in the cold water bath to produce potash alum. The crystals of potash alum are formed in the solution after a few hours. Analysis of the product potash alum, parent liquor and the residual liquor shows that nearly 89.2% of aluminium present in the parent liquor is successfully transferred into the product potash alum crystals. Potash alum finds its applications in wastewater treatment plants, pharmaceutical cosmetic industries and leather tanning units.
Similar content being viewed by others
1 Introduction
Nearly 58,890 thousand metric ton of aluminium is produced annually, and it is projected that the production shall grow further in the upcoming years [1]. The aluminium production industry is now shifting its dominance to the secondary aluminium production. The melting and remelting of the aluminium ingots produced from primary aluminium industries and aluminium waste scraps are vital processing steps in delivering the final products in secondary aluminium industries.
In all of these processes, aluminium dross production takes place. An oxide film forms over the metallic aluminium, when the molten aluminium comes in contact with atmospheric oxygen. This oxide product, called aluminium dross, is manually removed away from the molten aluminium. It is essentially a mixture of metallic aluminium entrapped in alumina and salt fluxes used in smelter plants. Annually, around 1.5–2.5% and 8–15% of primary and secondary dross is produced per ton of molten metal [1, 2].
A large proportion of aluminium dross produced is landfilled, which is an improper way of disposing such a hazardous waste product. Disposing aluminium dross is an industrial concern for all the aluminium smelter units. The motivation of the present research is to illustrate a new method of recycling white aluminium dross. An insight of recycling methods described by various researchers is presented as follows.
Due to the presence of leachable salts in aluminium dross, it damages the local environment [3]. This affects the land resource where the landfilling is carried out [4]. Also, when aluminium dross containing aluminium nitride comes in contact with water, it produces ammonia gas and other hazardous gases [5].
Considering the total amount of aluminium dross produced annually and the potential hazards of the industrial waste material, the recycling of aluminium dross becomes the necessity of the day. Conventional recycling processes of aluminium dross include the pyrometallurgical and hydrometallurgical methods. The rotary salt furnaces and plasma arc furnaces consume aluminium dross as raw materials for the extraction of metallic aluminium, thereby leaving behind the non-metallic product, which primarily consists of alumina and salt fluxes.
The rotary salt furnaces consume salts like sodium chloride and potassium chloride to prevent further oxidation of molten aluminium and bring down the operation temperature [6, 7]. Processes like Hydro-Quebec DROSCAR graphite arc process, DC electric arc process and ALUREC process have been developed that depend on plasma arc, electric arc and oxy-fuel, respectively, for the extraction of metallic values from aluminium dross [8,9,10]. ECOCENT and PyroGenesis DROSRITE processes utilize the heat energy of the hot aluminium dross that is charged into the furnace. The tumbling action of the rotary furnace leads to the extraction of metallic aluminium and separation of non-metallic product [10, 11].
The hydrometallurgical route utilizes aluminium dross for leaching in acidic and alkaline media to generate various valuable products. Das et al. have used sulphuric acid for leaching nearly 84% Al2O3 into the liquor in 3 h from white aluminium dross at 363 K [12]. Similarly, Tsakiridis et al. have used sodium hydroxide solution to leach around 57% aluminium into the basic liquor in 2 h from black dross at the temperature range of 433–533 K [13].
Various new methods for recycling of aluminium dross have been developed recently. These include the generation of valuable products like layered double hydrates, zeolites, ion exchangers and using aluminium dross as the raw material [14, 15]. The utilization of metallic aluminium entrapped in the matrix of alumina is achieved by using these methods.
Using aluminium dross as a raw material, the authors have produced a relatively rare mineral, tamarugite, which has good coagulant properties [16]. The leaching of aluminium dross has been carried out using sodium hydroxide, and subsequent addition of sulphuric acid led to the crystallization of tamarugite, NaAl(SO4)2.6H2O. Also, the authors have examined the evolution of hydrogen gas when it comes in contact with water [17,18,19].
In this article, the authors suggest a new method to recycle waste white aluminium dross by utilizing its metallic aluminium content to produce potash alum. Waste aluminium foils and medical wastes containing aluminium have been used for the production of potash alum, as illustrated by various researchers [20, 21]. The main objectives of this research article are: the recycling of white aluminium dross (as it is a hazardous industrial by-product) and the simultaneous production of potash alum.
Potash alum is a very important inorganic compound that has many commercial applications. It is mainly used in wastewater treatment plants as a chemical flocculant to remove the suspended colloids present in the turbid water [22]. The other beneficial applications of potash alum are leather tanning and fabric dyeing, apart from being a necessary ingredient in medicines and cosmetic products [23,24,25].
Conventionally, potassium aluminium sulphate, KAl(SO4)2.12H2O, is produced by crystallization from a solution that contains potassium sulphate and aluminium sulphate. Aluminium sulphate is produced by reacting bauxite with sulphuric acid. Many scientists have investigated the crystallization phenomenon of potash alum and its astonishing applications in the medicinal and wastewater purification front.
Birnin-Yauri et al. and Ekere et al. have produced crystals of potash alum from used aluminium beverage cans and aluminium scraps. The beverage cans and scraps were dissolved in concentrated potassium hydroxide solution, after which the solution was subjected to react with sulphuric acid, followed by boiling and cooling [21, 26]. The crystals produced from the solution were used for coagulation test.
Kardos et al. have synthesized spherical agglomerates of potash alum from its crystals, which can be used for improving the solubility of drugs in water. For this purpose, potash alum crystals were dissolved in water and spherical agglomerates were precipitated from the solution using a mixture of organic solvents [27]. Similarly, Gupta et al. used potash alum as a catalyst for the production of furfural, 5-hydroxymethylfurfural and levulinic acid, which are important chemicals for biofuel [28].
In the present world of pressing economic demands and environmental crises, discovering newer methods and processes to recycle waste hazardous aluminium dross becomes the only viable option for the scientific community. It is fresh dimension of waste utilization research, which can be further explored. In the next sections, the processing of white aluminium dross is illustrated, along with the production and characterization of potash alum. The essence of the article is the industrial waste management to form important product.
2 Experimental
Aluminium dross was procured from Deva Metal Powders Pvt Ltd., Varanasi, India. As-received bulk aluminium dross sample was crushed and downsized using jaw crushers. The powdered aluminium dross was sieved, and fine particles (− 44 + 100 mesh size) were used for further experiments. The phases present in powdered aluminium dross and their relative amounts were determined using X-ray diffraction analysis, and the morphology of the particles was examined by scanning electron microscopy.
Sulphuric acid (Molychem), potassium hydroxide (SRL), EDTA disodium hydrate (SRL) and Eriochrome Black T (SRL) were used for the production and analysis of potash alum crystals. Borosil glassware and deionized water were used for the preparation of solutions and conducting the experiments.
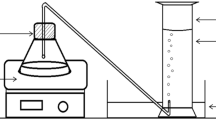
Figure 1 shows the experimental procedure for the production of potash alum from the powdered aluminium dross. Two grams of fine aluminium dross powder was added to 50 mL of 3 M potassium hydroxide solution at 45 °C for nearly 60 min. This solution was filtered to remove the residue, and a clear filtrate containing the complex oxide product was obtained. A solution of 3 M H2SO4 was prepared for the addition of sulphate ions to the complex oxide product formed above to produce potash alum. Seventy millilitres of H2SO4 was added to the solution filtrate slowly using burette. Continuous stirring of the solution using a glass rod was carried out throughout the process of addition of H2SO4. White gelatinous precipitate of aluminium hydroxide, Al(OH)3, formed just as the drops of H2SO4 were mixed with the filtrate solution. As 70 mL of H2SO4 was added completely to the filtrate, the solution turned completely transparent [26].
This solution was boiled for 5 min, 8 min, 12 min and 15 min which turned the solution clear. This step was carried out to boil off excess acid from the solution to make it saturated. Variation of boiling time was performed to study the effect on the overall mass produced of potash alum. After sufficient boiling, the beaker containing the solution was quickly transferred to a chilled water bath. Nota bene that the experiments were repeated four times and the average values of mass produced were reported. The beaker was left undisturbed for nearly 20 h.
After the crystallization of potash alum at the bottom of the beaker, the liquor was decanted to separate the crystals from the solution and placed in the chilled water bath again. This led to crystallization of alum after primary crop. The total crop of alum crystals was collected and stored in a desiccator. The overall amount of alum was measured using a digital balance (OHAUS, USA). The alum crystals were dissolved in distilled water, recrystallized and washed with 10% v/v ethanol solution to remove any impurity associated with it and protect the final crop from contamination. To confirm the potash alum phase in the crop, X-ray diffraction analysis was carried out using Rigaku Tabletop MiniFlex 600, Dtex Ultra, with copper target Cu Kα = 1.54 Ǻ, while the morphology of the crystals of potash alum was examined by scanning electron microscope (Zeiss Evo-18 Research 2045, Oxford X-act INCA x-act).
Aluminium content of alum and liquor (before and after the alum production) is determined using complexometric EDTA back titration method, with Eriochrome Black-T indicator. The details of the complexometric back titration experimental procedure can be found in the literature [29]. Right after the reaction of aluminium dross powder and potassium hydroxide, the filtered liquor obtained was collected for titration. Similarly, potash alum samples were dissolved in deionized water, and the solution was analysed for the amount of aluminium present in it. Finally, the residual liquor obtained after the production of potash alum crystals was analysed.
ICP analysis of potash alum has been carried out to examine the elemental composition of the resultant product. TGA–DTA was carried out for potash alum using TGA-50 Shimadzu Thermogravimetric Analyser. Nitrogen atmosphere was used under gas flow of 50 mL per minute. Platinum crucibles were used, and rate of heating was 10 °C per minute. The temperature range was 30 °C to 500 °C, and the initial mass of the alum sample was 7.18199 mg.
3 Results and Discussions
3.1 Characterization of Aluminium Dross
From the X-ray diffraction pattern of the powdered aluminium dross shown in Fig. 2.a, it can be understood that it has aluminium, alumina and silica phases (PCPDF file no: 89-4037, 47-1292 and 88-2487). The quantification of phases has been carried out by calculating the integrated area under the peaks of the phases. The approximate amounts of the phases are shown in Table 1. It is clear that aluminium is dominant phase in the powdered aluminium dross, whereas silica and alumina are present in smaller amounts.
As can be seen from the SEM image of aluminium dross powder in Fig. 2.b, the particles observed are of asymmetric morphology with varied particle size. The EDS report of the particles shows the presence of aluminium and oxygen (Fig. 2.c). An observation of XRD pattern and SEM–EDS report of aluminium dross reveals the dominant presence of aluminium in the dross. This metallic content is recycled by subjecting the white aluminium dross to reaction with potassium hydroxide and sulphuric acid to produce potash alum.
3.2 Reaction Progression for Production of Potash Alum
According to the literature, when aluminium comes in contact with potassium hydroxide solution, the formation of Al(OH)−4 ions takes place [26]. This combines with the potassium ions present in the solution. The same is observed when aluminium dross reacts with potassium hydroxide solution. The formation of hydrogen gas is marked. Potassium tetrahydroxoaluminate (III), KAl(OH)4, is the product formed in the solution. Equations (1) and (2) show the reactions that take place [26]:
This solution when titrated slowly against H2SO4, the formation of K2SO4 and removal of OH− ions from K[Al(OH)4] take place, thereby precipitating white gelatinous aluminium hydroxide. This aluminium hydroxide when reacts with H2SO4 produces aluminium sulphate. The reactions that take place are shown in Eqs. (3)–(5) [26]:
Excess amount of H2SO4 is added to the solution to induce crystallization in accordance with Le Chatelier’s principle. The value of pH of the leached liquor before the addition of sulphuric acid is nearly 13. As the drops of acid are introduced into the solution, the pH gradually drops and finally it reaches a value of 1. At this point, aluminium sulphate and potassium sulphate formed in the solution lead to the precipitation of potash alum crystals. This is shown in Eq. (6) [26]:
Although white aluminium dross contains inherent impurities and multiple phases, it acts as a raw material for the formation of potash alum. The resultant crop is recrystallized to achieve a purer product, removing impurities present in it. Figure 3 illustrates the mechanism of reactions for the formation of potash alum.
3.3 Underlying Mass Flow of Aluminium During the Production of Potash Alum
It is observed from the schematic of mass flow (Fig. 4) that nearly 62.5% of mass of aluminium dross successfully gets transferred into the liquor, whereas 37.5% of aluminium dross remains unreacted and forms the content for residual solid. It primarily contains SiO2 and some unreacted aluminium and aluminium phases. Taking the amount of aluminium that gets transferred into the primary liquor before the alum production as 100%, the aluminium content of the alum and the residual liquor are calculated accordingly.
The potash alum produced is dissolved in deionized water, and the total amount of aluminium present in the solution is determined (89.2% of the total amount of aluminium in the parent liquor). The residual liquor produced after the crystallization shows significantly less amount of aluminium (nearly 10.8% of the total aluminium present in the parent liquor). The residual liquor becomes highly acidic, the pH of the solution being close to 1. Nota bene that the characterization experiments using EDTA back titration are repeated four times to ensure the authenticity of the result.
An interesting point is the total amount of aluminium present in the potash alum produced experimentally. The aluminium content of commercial potash alum (20.90 g) is 1.19 g, whereas the amount of aluminium in 20.9 g of experimentally produced potash alum using EDTA back titration is 1.11 g.
3 M, 50 mL KOH (0.15 mol of KOH) was reacted with fixed amount of white aluminium dross containing aluminium and alumina to give 0.0462 mol of potassium aluminate K[Al(OH)4] as confirmed by the EDTA-EBT back titrations. This potassium aluminate is reacted with excess sulphuric acid (3 M, 70 mL) to give mixture of two soluble salts, aluminium sulphate Al2(SO4)3 (aq.) and potassium sulphate K2SO4 (aq.). The stoichiometric ratio (from Eqs. (4–5)) of potassium aluminate, K[Al(OH)4], and aluminium sulphate, Al2(SO4)3, is 2:1. Therefore, for 0.0462 mol of potassium aluminate, 0.0231 mol of aluminium sulphate will be produced.
According to Eq. (6), for 1 mol of potash alum, 0.5 mol of aluminium sulphate is required. When 0.0231 mol of aluminium sulphate reacts with potassium sulphate, 0.0462 mol of potash alum (21.89 g) should be theoretically produced. However, it is found experimentally that 0.044 mol (20.9 g) of potash alum is produced. Thus, the efficiency of potash alum generation in the experiment is 95.47% in light of the theoretically expected value.
Table 2 shows the amount of alum prepared after varying the boiling time. The amount of alum produced depends on the time period of boiling allowed for saturating the solution. It can be seen that insufficient boiling leads to reduction in the amount of alum produced. When the boiling time is varied from 5 to 15 min, the amount of alum produced is nearly doubled. A glass rod is introduced into the beaker to check the crystallization point of the solution in every experiment. It is observed that crystallization is spontaneous when the boiling time period is 15 min, whereas in other cases, the crystallization on the glass rod is not evident. As expected, the solution with higher saturation results in higher final crop. Heterogeneous nucleation and growth of the nuclei resuls in the crystallization of potash alum. The boiling of the solution is then stopped, and the beaker is quickly transferred to chilled water bath. The formation of alum crystals is observed to begin at the bottom and the walls of the beaker.
3.4 Characterization of Potash Alum
X-ray diffraction pattern for potash alum is depicted in Fig. 5a. Potash alum has simple cubic structure. As can be seen from the pattern, the alum produced is fairly pure (PCPDF file no: 71-2201). The major peaks are of planes (220), (321), (221) and (331). SEM–EDS of potash alum is depicted in Fig. 5b, c.
The SEM image of potash alum in Fig. 4b shows the crystallographic facets of the particles. The surface appears to be undulated, and the particle size is around 200–500 μm. The presence of aluminium, potassium, sulphur and oxygen is confirmed by the EDS report.
To further examine the elemental composition of potash alum, ICP-OES has been done. It is found that the sample has around 1.046 mg/L potassium ions and 0.533 mg/L aluminium ions. According to the stoichiometry of potash alum, the amount of potassium should be twice the amount of aluminium, which is confirmed by the ICP analysis.
The thermogravimetric analysis of potash alum is shown in Fig. 6. The linear fitting is performed on Origin software over different temperature ranges to get an approximation of transition temperatures. The transition temperatures identified are 73 °C, 117 °C, 231 °C and 241 °C. The mass loss begins at 73 °C and ends at 241 °C because the value of mass becomes almost constant as observed in the thermogravitogram. The TGA plot is in accordance with the literature [30].
The mass loss of potash alum corresponding to the temperature range of 73–241 °C is 3.16 mg. This mass loss is the loss of water of crystallization associated with the potash alum crystals. The number of molecules of water of crystallization (calculated from the mass loss) is found to be 11.89, which is very close to the actual value of water molecules present in potash alum (no. of water molecules associated = 12). Differential thermal analysis of alum samples show two endothermic peaks at 95 °C and at 234 °C with respect to baseline. These endotherms are corresponding to decomposition of water of crystallization.
It is seen that 37.5% of aluminium dross consumed for the production is separated as solid residue. It is necessary to characterize this solid residue. Figure 7 illustrates the XRD pattern of the solid residue. It can be seen that the phases identified in the pattern are bayerite, silica and nordstrandite. The silica present in the dross has remained in the residual solid. The utilization of the residual solid can be done to produce alumina, which has its own applications [18]. Aluminium–alumina composites employ alumina as reinforcement; the residual solid can be incorporated as a raw material for the production of the composites.
Figure 8 abridges the essence of the research article by depicting the utilization of aluminium dross. The major utilization of aluminium dross has been to extract metallic aluminium from it by means of pyrometallurgical route; other applications include production of cement and concrete, ion exchangers, composites, zeolites and refractory materials. When aluminium dross is used for the production of potash alum, it will, progressively, be beneficial to wastewater treatment plants, leather tanning units, fabric dyeing, pharmaceutical and cosmetic industries.
4 Conclusions
The method discussed in this article is an alternative means of recycling aluminium dross and utilizing the metallic aluminium entrapped in it. The analyses through characterization techniques like XRD, SEM–EDS, ICP-OES, TGA and DTA clearly indicate that the potash alum produced from white aluminium dross has appreciably good quality. The XRD pattern shows the single phase of potash alum, and SEM–EDS depicts the undulated morphology and elemental analysis. ICP-OES further confirms the elemental composition and the stoichiometry of the resultant product. TGA-DTA describes the thermal decomposition and the amount of water of crystallization associated with potash alum. Around 20.9 g of potash alum can be produced by using 2 g of white aluminium dross. Potash alum, a commercially important chemical, produced through this technique can be used in water treatment plants and other industries. Generation of valuable products from such waste materials is an exciting aspect of industrial waste management, which can further reduce the environmental burden.
References
Meshram A, and Singh K K, Resour Conserv Recycl130 (2018) 95.
Mankhand T R, J Sustain planet3 (2012) 86.
Shinzato M C, and Hypolito R, Environ Earth Sci75 (2016) 628.
Gil A, Environ Eng Sci24 (2007) 1234.
Peng L, Zhang M, Teng L, et al, Metall Mater Trans B44 (2013) 16.
Soares Tenorio J A, and Romano Espinosa D C, J Light Met2 (2002) 89.
Masson D B, and Taghiei M M, Mater Trans30 (1989) 411.
Tzonev T, and Lucheva B, Jom64 (2007).
Drouet M G, Handfield M, Meunier J, et al, Jom26 (1994).
Ünlü N, andDrouet M G, Resour Conserv Recycl36 (2002) 61.
Drouet MG, Leroy RL, Tsantrizos PG. Drosrite Salt-Free Processing Of Hot Aluminum Dross. Pittsburgh, Pennsylvania, 2000, pp. 1135–1145.
Das B R, Dash B, Tripathy B C, et al, Miner Eng252 (2007).
Tsakiridis P E, Oustadakis P, and Agatzini-Leonardou S, J Environ Chem Eng1 (2013) 23.
Murayama N, Int J Miner Process110–111 (2012) 46.
Hiraki T, Nosaka A, Okinaka N, et al, Iron Steel Inst Japan Int49 (2009) 1644.
Meshram A, Jain A, Gautam D, et al, J Environ Manage232 (2019) 978.
Meshram A, and Singh K K, Generation of hydrogen-gas from aluminum dross. In: European Metallurgical Conference 2017. Leipzig, Germany, 2017, pp. 1451–1460.
Meshram A, Jain A, Rao M D, et al.,J Mater Cycles Waste Manag (2019).
Singh K K, Meshram A, Gautam D, et al, Agron Res. https://doi.org/10.15159/AR.19.022
Ugwekar R P, and Lakhawat G P, IOSR J Eng2 (2012) 62.
Ekere N R, Ihedioha J N, and Bright A A, Int J Chem Sci12 (2014) 1145.
Mukherjee S, Mukhopadhyay S, Pariatamby A, et al, J Environ Sci26 (2014) 1851.
Alzomor A K, Moharram A S, and Al Absi N M, Int Curr Pharm J3 (2014) 228.
Priya T, Sindhu S K, and Gautam V S, Asian J Bio Sci8 (2013) 129.
Ahmed Z, Afzal M, Kazmi I, et al, Pharmacol Biochemi Approach3 (2012) 729.
Birnin-Yauri A U, and Aliyu M, Int J Adv Res Chem Sci1 (2014) 1.
Kardos A F, Tóth J, Trif L, et al, RSC Adv6 (2016) 5466.
Gupta D, Ahmad E, Pant K K, et al, RSC Adv7 (2017) 41973.
Jeffery G H, Bassett J, Mendham J, et al, Vogel’s Textbook of Quantitative Chemical Analysis. Fifth Edit. New York: Longman Scientific & Technical. Epub ahead of print 1989. https://doi.org/10.1016/0160-9327(90)90087-8.
Souza R, Navarro R, Grillo A V, et al, J Mater Res Technol8 (2018) 745.
Acknowledgements
The authors express their deep reverence to the Head, Department of Metallurgical Engineering, IIT (BHU), for providing the facilities essential for conducting the experiments. Also, the authors are thankful to Mr. Amitabh Deva, MD, Deva Metal Powders Pvt., Ltd., for providing aluminium dross. Acknowledgement is extended to Dr. Bratindranath Mukherjee, Assistant Professor, IIT (BHU), Mr. Seby Varghese and technical staff of Extractive Metallurgy Division, Department of Metallurgical Engineering, Indian Institute of Technology, Banaras Hindu University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meshram, A., Gautam, D. & Singh, K.K. Recycling of White Aluminium Dross: Production of Potash Alum. Trans Indian Inst Met 73, 1239–1248 (2020). https://doi.org/10.1007/s12666-020-01973-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-01973-1