Abstract
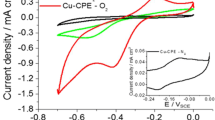
Hydrogen peroxide (H2O2) is one of the most popular and widely used oxidants. Among the wide range of synthesis techniques used for the production of H2O2; the electrochemical method allows the use of gas diffusion electrodes to generate H2O2 without limiting the low solubility of O2 in water. The present work reports the modification of carbon black with quinone, where the generation of H2O2 occurs in an electrochemical/chemical mechanism. The results obtained by this technique were found to be highly promising. The use of the organic compound 1,2-dihydroxyanthraquinone to modify carbon black electrode resulted in greater production of H2O2. Carbon black electrode modified with 1% of 1,2-dihydroxyanthraquinone yielded 298 mg L−1 of H2O2 at the end of 90 min of experiment, reaching an electrical efficiency of approximately 25.5%. Based on the findings of this study, H2O2 generation is found to be directly associated with the chemical structure of the carbon modifier and not solely related to the presence of quinone groups.

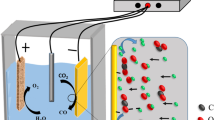
Graphical Abstract





Similar content being viewed by others
References
S. Ranganathan, V. Sieber, Catalysts 8, 379 (2018)
R.B. Valim, R.M. Reis, P.S. Castro, A.S. Lima, R.S. Rocha, M. Bertotti, M.R.V. Lanza, Carbon 61, 236 (2013)
J.M. Campos-Martin, G. Blanco-Brieva, J.L.G. Fierro, Angew. Chem. Int. 45, 6962 (2006)
E.L. Gyenge, C.W. Oloman, J. Appl. Electrochem. 33, 655 (2003)
D. Bao, S. Ramu, A. Contreras, S. Upadhyayula, J.M. Vasquez, G. Beran, V.I. Vullev, J. Phys. Chem. B 114, 14467 (2010)
J.-S. Do, C.-P. Chen, J. Appl. Electrochem. 24, 936 (1994)
D. Alvarez-Gallegos, Pletcher. Electrochim. Acta 44, 853 (1998)
C. Badellino, C.A. Rodrigues, R. Bertazzoli, J. Appl. Electrochem. 37, 451 (2007)
S.C. Perry, D. Pangotra, L. Vieira, L.-I. Csepei, V. Sieber, L. Wang, C.P. de Leon, F.C. Walsh, Nat. Rev. Chem 3, 442 (2019)
E. Brillas, M.A. Banos, S. Camps, C. Arias, P.L. Cabot, P.L. Garrido, J.A.R.M. Rodríguez, New J. Chem. 28, 314 (2004)
H.W. Kim, M.B. Ross, N. Kornienko, L. Zhang, J. Guo, P. Yang, B.D. McCloskey, Nat. Catalysis. 1, 282 (2018)
C.A.R. Ragnini, R.A. Di Iglia, R. Bertazzoli, Quim Nova 24, 252 (2001)
M. Giomo, A. Buso, P. Fier, B. Boye, G.A. Farnia, Electrochim Acta 54, 808 (2008)
M.H.M.T. Assumpção, A. Moraes, R.F.B. De Souza, I. Gaubeur, R.T.S. Oliveira, V.S. Antonin, G.R.P. Malpass, R.S. Rocha, M.L. Calegaro, M.R.V. Lanza, M.C. Santos, Appl. Catal. A Gen. 411-412, 1 (2012)
R. Abdel-Hamid, M.K. Rabia, H.M. El-Sagher, Bull. Chem. Soc. Jpn. 70, 2389 (1997)
S. Komorsky-Lovric, J. Anal. Chem. 356, 306 (1996)
X.-S. Chai, Q.X. Hou, Q. Luo, J.Y. Zhu, Anal. Chim. Acta 507, 281 (2004)
P.S. Guin, S. Das, P.C. Mandal, Int. J. Electrochem. 189, 206 (2011)
J.F. Carneiro, R.S. Rocha, P. Hamer, R. Bertazzoli, M.R.V. Lanza, Appl. Catal. A: General. 517, 161 (2016)
L.R. Radovic, A.J.A. Salgado-Casanova, Carbon. 126, 443 (2018)
A.M.O. Brett, C.M.A. Brett, Electrochemistry: Principles, Methods and Applications, 5nd edn. (Oxford University Press 1996), p. 273
M.H.M.T. Assumpção, R.F.B. De Souza, D.C. Rascio, J.C.M. Silva, M.L. Calegaro, I. Gaubeur, T.R.L.C. Paixão, P. Hammer, M.R.V. Lanza, M.C. Santos, Carbon. 49, 2842 (2011)
K. Tammeveski, K. Kontturi, R.J. Nichols, R.J. Potter, D.J. Schiffrin, J. Electroanal. Chem. 515, 101 (2001)
P. Huissoud, J. Tissot, Appl. Electrochem. 28, 653 (1998)
F. Wang, S. Hu, Electrochim. Acta 51, 4228 (2006)
Y. Feng, T. He, N.A. Vante, Electrochim. Acta 54, 5252 (2009)
E. Roche, M. Chainet, J. Chatenet, J. Vondrak, Phys. Chem. C 111, 1434 (2007)
R.M. Reis, A.A.G.F. Beati, R.S. Rocha, M.H.M.T. Assumpção, M.C. Santos, R. Bertazzoli, M.R.V. Lanza, Ind. Eng. Chem. Res. 51, 649 (2012)
Funding
The authors are sincerely grateful to the Brazilian research funding agencies, including the Brazilian National Council for Scientific and Technological Development – CNPq (grants no. 465571/2014-0, 302874/2017-8 and 427452/2018-0), São Paulo Research Foundation (FAPESP – grants #2011/14314-1, #2016/22115-2, #2014/50945-4, #2013/02762-5, #2016/12597-0, #2019/00239-0 and #2017/10118-0), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Finance Code 001) for the financial support granted in the course of this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rocha, R.S., Valim, R.B., Trevelin, L.C. et al. Electrocatalysis of Hydrogen Peroxide Generation Using Oxygen-Fed Gas Diffusion Electrodes Made of Carbon Black Modified with Quinone Compounds. Electrocatalysis 11, 338–346 (2020). https://doi.org/10.1007/s12678-020-00591-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-020-00591-1