Abstract
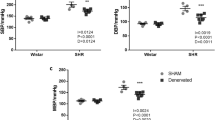
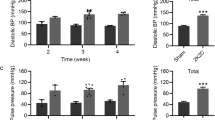
Vasoconstriction within the renal medulla contributes to the development of hypertension. This study investigated the role of reactive oxygen species (ROS) in regulating renal medullary and cortical blood perfusion (MBP and CBP respectively) in both stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar rats. CBP and MBP were measured using a laser-Doppler flow meter before and after intra-renal infusion of tempol, the superoxide dismutase (SOD) mimetic or tempol plus catalase, the hydrogen peroxide-degrading enzyme. Tempol infusion significantly elevated blood perfusion within the renal medulla (MBP) in both SHRSP (by 43 ± 7%, P < 0.001) and Wistar rats (by 17 ± 2%, P < 0.05) but the magnitude of the increase was significantly greater in the SHRSP (P < 0.01). When the enzyme catalase and tempol were co-infused, MBP was again significantly increased in SHRSP (by 57 ± 6%, P < 0.001) and Wistar rats (by 33 ± 6%, P < 0.001), with a significantly greater increase in perfusion being induced in the SHRSP relative to the Wistar rats (P < 0.01). Notably, this increase was significantly greater than in those animals infused with tempol alone (P < 0.01). These results suggest that ROS plays a proportionally greater role in reducing renal vascular compliance, particularly within the renal medulla, in normotensive and hypertensive animals, with effects being greater in the hypertensive animals. This supports the hypothesis that SHRSP renal vasculature might be subjected to elevated level of oxidative stress relative to normotensive animals.




Similar content being viewed by others
References
Ahmeda AF, Johns EJ (2012) The regulation of blood perfusion in the renal cortex and medulla by reactive oxygen species and nitric oxide in the anaesthetised rat. Acta Physiol (Oxf) 204(3):443–450
Ahmeda AF, Rae MG, Johns EJ (2013) Effect of reactive oxygen species and nitric oxide in the neural control of intrarenal haemodynamics in anaesthetized normotensive rats. Acta Physiol (Oxf) 209(2):156–166
Ardanaz N, Pagano PJ (2006) Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med 231(3):237–251
Armstrong JM, Blackwell GJ, Flower RJ, McGiff JC, Mullane KM, Vane JR (1976) Genetic hypertension in rats is accompanied by a defect in renal prostaglandin catabolism. Nature 260(5552):582–586
Beckman JS, Crow JP (1993) Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans 21(2):330–334
Berecek KH, Schwertschlag U, Gross F (1980) Alterations in renal vascular resistance and reactivity in spontaneous hypertension of rats. Am J Phys 238(3):H287–H293
Bouloumie A, Bauersachs J, Linz W, Scholkens BA, Wiemer G, Fleming I, Busse R (1997) Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension 30(4):934–941
Burke-Wolin T, Abate CJ, Wolin MS, Gurtner GH (1991) Hydrogen peroxide-induced pulmonary vasodilation: role of guanosine 3',5'-cyclic monophosphate. Am J Phys 261(6 Pt 1):L393–L398
Chen YF, Cowley AW Jr, Zou AP (2003) Increased H(2)O(2) counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol Regul Integr Comp Physiol 285(4):R827–R833
Cosentino F, Katusic ZS (1995) Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation 91(1):139–144
Cosentino F, Patton S, d’Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, Malinski T, Luscher TF (1998) Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest 101(7):1530–1537
Cowley AW Jr, Mattson DL, Lu S, Roman RJ (1995) The renal medulla and hypertension. Hypertension 25(4 Pt 2):663–673
Darley-Usmar V, Wiseman H, Halliwell B (1995) Nitric oxide and oxygen radicals: a question of balance. FEBS Lett 369(2–3):131–135
de Richelieu LT, Sorensen CM, Holstein-Rathlou NH, Salomonsson M (2005) NO-independent mechanism mediates tempol-induced renal vasodilation in SHR. Am J Physiol Renal Physiol 289(6):F1227–F1234
Dohi Y, Thiel MA, Buhler FR, Luscher TF (1990) Activation of endothelial L-arginine pathway in resistance arteries. Effect of age and hypertension. Hypertension 16(2):170–179
Feng MG, Dukacz SA, Kline RL (2001) Selective effect of tempol on renal medullary hemodynamics in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 281(5):R1420–R1425
Fujimoto S, Asano T, Sakai M, Sakurai K, Takagi D, Yoshimoto N, Itoh T (2001) Mechanisms of hydrogen peroxide-induced relaxation in rabbit mesenteric small artery. Eur J Pharmacol 412(3):291–300
Gao YJ, Hirota S, Zhang DW, Janssen LJ, Lee RM (2003) Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol 138(6):1085–1092
Gao YJ, Zhang Y, Hirota S, Janssen LJ, Lee RMKW (2004) Vascular relaxation response to hydrogen peroxide is impaired in hypertension. Br J Pharmacol 142(1):143–149
Gil-Longo J, Gonzalez-Vazquez C (2005) Characterization of four different effects elicited by H2O2 in rat aorta. Vasc Pharmacol 43(2):128–138
Grisk O, Kloting I, Exner J, Spiess S, Schmidt R, Junghans D, Lorenz G, Rettig R (2002) Long-term arterial pressure in spontaneously hypertensive rats is set by the kidney. J Hypertens 20(1):131–138
Grunfeld S, Hamilton CA, Mesaros S, McClain SW, Dominiczak AF, Bohr DF, Malinski T (1995) Role of superoxide in the depressed nitric oxide production by the endothelium of genetically hypertensive rats. Hypertension 26(6 Pt 1):854–857
Harrison DG (1997) Endothelial function and oxidant stress. Clin Cardiol 20(11 Suppl 2):II-11-7
Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA (1999) Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension 33(6):1353–1358
Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A (1992) Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci U S A 89(12):5537–5541
Lacy F, Kailasam MT, O’Connor DT, Schmid-Schonbein GW, Parmer RJ (2000) Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36(5):878–884
Lominadze D, Joshua IG, Schuschke DA (1997) In vivo platelet thrombus formation in microvessels of spontaneously hypertensive rats. Am J Hypertens 10(10 Pt 1):1140–1146
Lote, C.J. (2000) Principles of renal physiology. Springer.
Lucchesi PA, Belmadani S, Matrougui K (2005) Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens 23(3):571–579
Makino A, Skelton MM, Zou AP, Cowley AW Jr (2003) Increased renal medullary H2O2 leads to hypertension. Hypertension 42(1):25–30
Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A (2000) Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106(12):1521–1530
Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A (2002) Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 290(3):909–913
Nagaoka A, Kakihana M, Suno M, Hamajo K (1981) Renal hemodynamics and sodium excretion in stroke-prone spontaneously hypertensive rats. Am J Phys 241(3):F244–F249
Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y (2001) Systemic and regional hemodynamic responses to tempol in angiotensin II-infused hypertensive rats. Hypertension 37(1):77–83
Okamoto K, Aoki K (1963) Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27:282–293
Okamoto K, Yamori Y, Nagaoka A (1974) Establishment of the stroke-prone spontaneously hypertensive rat (SHR). Circ Res 34(35):143–153
Omar HA, Figueroa R, Omar RA, Tejani N, Wolin MS (1992) Hydrogen peroxide and reoxygenation cause prostaglandin-mediated contraction of human placental arteries and veins. Am J Obstet Gynecol 167(1):201–207
Park JB, Touyz RM, Chen X, Schiffrin EL (2002) Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens 15(1 Pt 1):78–84
Pelaez NJ, Braun TR, Paul RJ, Meiss RA, Packer CS (2000) H(2)O(2) mediates Ca(2+)- and MLC(20) phosphorylation-independent contraction in intact and permeabilized vascular muscle. Am J Physiol Heart Circ Physiol 279(3):H1185–H1193
Rhoades RA, Packer CS, Meiss RA (1988) Pulmonary vascular smooth muscle contractility. Effect of free radicals. Chest 93(3 Suppl):94S–95S
Rodriguez-Martinez MA, Garcia-Cohen EC, Baena AB, Gonzalez R, Salaices M, Marin J (1998) Contractile responses elicited by hydrogen peroxide in aorta from normotensive and hypertensive rats. Endothelial modulation and mechanism involved. Br J Pharmacol 125(6):1329–1335
Schnackenberg CG (2002) Oxygen radicals in cardiovascular-renal disease. Curr Opin Pharmacol 2(2):121–125
Schnackenberg CG, Wilcox CS (1999) Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension 33(1 Pt 2):424–428
Schnackenberg CG, Welch WJ, Wilcox CS (1998) Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32(1):59–64
Shen JZ, Zheng XF, Kwan CY (2000) Differential contractile actions of reactive oxygen species on rat aorta: selective activation of ATP receptor by H2O2. Life Sci 66(21):PL291–PL296
Skov K, Mulvany MJ, Korsgaard N (1992) Morphology of renal afferent arterioles in spontaneously hypertensive rats. Hypertension 20(6):821–827
Stankevicius E, Martinez AC, Mulvany MJ, Simonsen U (2002) Blunted acetylcholine relaxation and nitric oxide release in arteries from renal hypertensive rats. J Hypertens 20(8):1571–1579
Suzuki H, Swei A, Zweifach BW, Schmid-Schonbein GW (1995) In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension 25(5):1083–1089
Szentivanyi M Jr, Maeda CY, Cowley W Jr (1999) Local renal medullary L-NAME infusion enhances the effect of long-term angiotensin II treatment. Hypertension 33(1 Pt 2):440–445
Tagami M, Nara Y, Kubota A, Sunaga T, Maezawa H, Fujino H, Yamori Y (1987) Ultrastructural characteristics of occluded perforating arteries in stroke-prone spontaneously hypertensive rats. Stroke 18(4):733–740
Taylor NE, Cowley AW Jr (2005) Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289(6):R1573–R1579
Tyther R, Ahmeda A, Johns E, Sheehan D (2009) Protein carbonylation in kidney medulla of the spontaneously hypertensive rat. Proteomics Clin Appl 3(3):338–346
Tyther R, Ahmeda A, Johns E, McDonagh B, Sheehan D (2010) Proteomic profiling of perturbed protein sulfenation in renal medulla of the spontaneously hypertensive rat. J Proteome Res 9(5):2678–2687
Wilcox CS (2002) Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep 4(2):160–166
Wilcox CS, Pearlman A (2008) Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 60(4):418–469
Xu H, Jackson WF, Fink GD, Galligan JJ (2006) Activation of potassium channels by tempol in arterial smooth muscle cells from normotensive and deoxycorticosterone acetate-salt hypertensive rats. Hypertension 48(6):1080–1087
Yang Z, Zhang A, Altura BT, Altura BM (1999) Hydrogen peroxide-induced endothelium-dependent relaxation of rat aorta involvement of Ca2+ and other cellular metabolites. Gen Pharmacol 33(4):325–336
Yang ZW, Zhang A, Altura BT, Altura BM (1998) Endothelium-dependent relaxation to hydrogen peroxide in canine basilar artery: a potential new cerebral dilator mechanism. Brain Res Bull 47(3):257–263
Yang ZW, Zheng T, Zhang A, Altura BT, Altura BM (1998) Mechanisms of hydrogen peroxide-induced contraction of rat aorta. Eur J Pharmacol 344(2–3):169–181
Zembowicz A, Hatchett RJ, Jakubowski AM, Gryglewski RJ (1993) Involvement of nitric oxide in the endothelium-dependent relaxation induced by hydrogen peroxide in the rabbit aorta. Br J Pharmacol 110(1):151–158
Zou AP, Cowley AW Jr (1997) Nitric oxide in renal cortex and medulla. An in vivo microdialysis study. Hypertension 29(1 Pt 2):194–198
Zou AP, Li N, Cowley AW Jr (2001) Production and actions of superoxide in the renal medulla. Hypertension 37(Part 2):547–553
Acknowledgments
We would like to thank the College of Medicine Research Centre (CMRC), Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia, for supporting the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
All procedures were performed in accordance with European Community Directive 2010/63/EU and were approved by University College Cork Animal Experimentation Ethics Committee.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ahmeda, A.F., Rae, M.G., Al Otaibi, M.F. et al. Effect of tempol and tempol plus catalase on intra-renal haemodynamics in spontaneously hypertensive stroke-prone (SHSP) and Wistar rats. J Physiol Biochem 73, 207–214 (2017). https://doi.org/10.1007/s13105-016-0541-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0541-1