Abstract
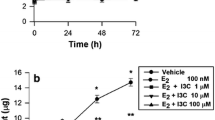
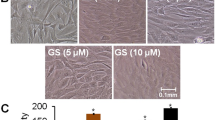
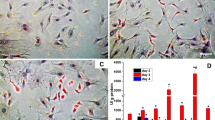
Phytoestrogens have been proposed as a natural therapy for prevention of bone loss. In this work, we studied the mechanism of action of genistein on osteoblast differentiation. Primary cell cultures of calvarial osteoblasts isolated from female Wistar rats were in vitro exposed to genistein. Osteoblast differentiation markers were measured. Genistein stimulated osteoblast migration (71–257% above control). An earlier upregulation of estrogen receptor alpha gene expression and an enhancement of mRNA levels of the Runt-related transcription factor 2 were detected after 3 days of culture. The isoflavone significantly increased osteocalcin expression, extracellular collagen deposition, and alkaline phosphatase activity. The mechanism displayed by genistein involved estrogen receptor and nitric oxide pathway participation, since cell preincubation with the estrogen receptor antagonist ICI 182780, or the nitric oxide synthase inhibitor L-NAME, suppressed the phytoestrogen action. Evidence of MAPK and PI3K transduction systems participation on the stimulatory action of genistein on extracellular collagen deposition and alkaline phosphatase activity was also obtained. Genistein favored monocyte adhesion to osteoblasts (77% above control) in an ER; NOS; and MAPK kinase–dependent and PI3K-dependent manner. Co-cultured osteoblast-monocyte long term exposed (21 days) to genistein exhibited a high number of multinucleated and tartrate-resistant acid phosphatase–positive cells added to osteoblasts, suggesting that the phytoestrogen promotes osteoclast differentiation. In conclusion, genistein promoted osteoblastogenesis through the participation of ER and NOS pathways, and the contribution of ERK or PI3K signal transduction pathways, and also stimulates osteoclast differentiation from its mononuclear progenitor.








Similar content being viewed by others
References
Abdi F, Alimoradi Z, Haqi P, Mahdizad F (2016) Effects of phytoestrogens on bone mineral density during the menopause transition: a systematic review of randomized, controlled trials. Climacteric 19(6):535–545. https://doi.org/10.1080/13697137.2016.1238451
Cepeda SB, Sandoval MJ, Rauschemberger MB, Massheimer VL (2017) Beneficial role of the phytoestrogen genistein on vascular calcification. J Nutr Biochem 50:26–37. https://doi.org/10.1016/j.jnutbio.2017.08.009
Cutini PH, Rauschemberger MB, Sandoval MJ, Massheimer VL (2016) Vascular action of bisphosphonates: in vitro effect of alendronate on the regulation of cellular events involved in vessel pathogenesis. J Mol Cell Cardiol 100:83–92. https://doi.org/10.1016/j.yjmcc.2016.08.017
De Gorter DJ, Ten Dijke P (2013) Signal transduction cascades controlling osteoblast differentiation. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, Clifford Rosen (Ed) Eighth Edition, Wiley-Blackwell, pp 15–41
Gao YH, Yamaguchi MASAYOSHI (2000) Suppressive effect of genistein on rat bone osteoclasts: involvement of protein kinase inhibition and protein tyrosine phosphatase activation. Int J Mol Med 5(3):261–268
Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, Arthur S, Xie M, Schneider MD, Zhai B, Gygi S, Davis R, Glimcher LH (2010) The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest 120(7):2457–2473. https://doi.org/10.1172/JCI42285
Han Y, Wang X, Ma D, Wu X, Yang P, Zhang J (2018) Ipriflavone promotes proliferation and osteogenic differentiation of periodontal ligament cells by activating GPR30/PI3K/AKT signaling pathway. Drug Design Develop Ther 12:137
Kalyanaraman H, Schall N, Pilz RB (2018) Nitric oxide and cyclic GMP functions in bone. Nitric Oxide 76:62–70. https://doi.org/10.1016/j.niox.2018.03.007
Kikuta J, Ishii M (2013) Osteoclast migration, differentiation and function: novel therapeutic targets for rheumatic diseases. Rheumatology (Oxford) 52(2):226–234. https://doi.org/10.1093/rheumatology/kes259
Komori T (2010) Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol 658:43–49. https://doi.org/10.1007/978-1-4419-1050-9_5
Liao QC, Xiao ZS, Qin YF, Zhou HH (2007) Genistein stimulates osteoblastic differentiation via p38 MAPK-Cbfa1 pathway in bone marrow culture. Acta Pharmacol Sin 28(10):1597–1602. https://doi.org/10.1111/j.1745-7254.2007.00632.x
Liao MH, Tai YT, Cherng YG, Liu SH, Chang YA, Lin PI, Chen RM (2014) Genistein induces oestrogen receptor-alpha gene expression in osteoblasts through the activation of mitogen-activated protein kinases/NF-kappaB/activator protein-1 and promotes cell mineralisation. Br J Nutr 111(1):55–63. https://doi.org/10.1017/S0007114513002043
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
McGarry JG, Klein-Nulend J, Prendergast PJ (2005) The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun 330(1):341–348. https://doi.org/10.1016/j.bbrc.2005.02.175
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108(1):17–29. https://doi.org/10.1016/s0092-8674(01)00622-5
O'Shaughnessy MC, Polak JM, Afzal F, Hukkanen MV, Huang P, MacIntyre I, Buttery LD (2000) Nitric oxide mediates 17beta-estradiol-stimulated human and rodent osteoblast proliferation and differentiation. Biochem Biophys Res Commun 277(3):604–610. https://doi.org/10.1006/bbrc.2000.3714
Papachristou DJ, Papachroni KK, Basdra EK, Papavassiliou AG (2009) Signaling networks and transcription factors regulating mechanotransduction in bone. Bioessays 31(7):794–804. https://doi.org/10.1002/bies.200800223
Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, Choi JY, Komori T, Stein JL, Lian JB, Stein GS, van Wijnen A (2003) Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res 63(17):5357–5362
Pratsinis H, Kletsas D (2007) PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J 16(11):1858–1866. https://doi.org/10.1007/s00586-007-0408-9
Pundt N, Peters MA, Wunrau C, Strietholt S, Fehrmann C, Neugebauer K, Seyfert C, van Valen F, Pap T, Meinecke I (2009) Susceptibility of rheumatoid arthritis synovial fibroblasts to FasL- and TRAIL-induced apoptosis is cell cycle-dependent. Arthritis Res Ther 11(1):R16. https://doi.org/10.1186/ar2607
Qi S, Zheng H (2017) Combined effects of phytoestrogen genistein and silicon on ovariectomy-induced bone loss in rat. Biol Trace Elem Res 177(2):281–287. https://doi.org/10.1007/s12011-016-0882-1
Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin W, Hu Y, Xu W, Xu L (2016) Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep 6:18732. https://doi.org/10.1038/srep18732
Sandoval MJ, Cutini PH, Rauschemberger MB, Massheimer VL (2010) The soyabean isoflavone genistein modulates endothelial cell behaviour. Br J Nutr 104(2):171–179. https://doi.org/10.1017/S0007114510000413
Shin S, Joung H (2013) A dairy and fruit dietary pattern is associated with a reduced likelihood of osteoporosis in Korean postmenopausal women. Br J Nutr 110(10):1926–1933. https://doi.org/10.1017/S0007114513001219
Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B (2006) Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab 91(4):1261–1267
Stenbeck G, Horton MA (2004) Endocytic trafficking in actively resorbing osteoclasts. J Cell Sci 117(Pt 6):827–836. https://doi.org/10.1242/jcs.00935
Tullberg-Reinert H, Jundt G (1999) In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem Cell Biol 112(4):271–276
Valiente M, Marín O (2010) Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol 20(1):68–78
Wang J, Li SF, Wang T, Sun CH, Wang L, Huang MJ, Chen J, Zheng SW, Wang N, Zhang YJ, Chen TY (2017) Isopsoralen-mediated suppression of bone marrow adiposity and attenuation of the adipogenic commitment of bone marrow-derived mesenchymal stem cells. Int J Mol Med 39(3):527–538. https://doi.org/10.3892/ijmm.2017.2880
Wang C, Zhang C, Zhou F, Gao L, Wang Y, Wang C, Zhang Y (2018) Angiotensin II induces monocyte chemoattractant protein1 expression by increasing reactive oxygen speciesmediated activation of the nuclear factorkappaB signaling pathway in osteoblasts. Mol Med Rep 17(1):1166–1172. https://doi.org/10.3892/mmr.2017.7971
Wimalawansa SJ (2010) Nitric oxide and bone. Ann N Y Acad Sci 1192:391–403. https://doi.org/10.1111/j.1749-6632.2009.05230.x
Wiren KM, Chapman Evans A, Zhang XW (2002) Osteoblast differentiation influences androgen and estrogen receptor-alpha and -beta expression. J Endocrinol 175(3):683–694
Wu Y, Xia L, Zhou Y, Xu Y, Jiang X (2015) Icariin induces osteogenic differentiation of bone mesenchymal stem cells in a MAPK-dependent manner. Cell Prolif 48(3):375–384
Acknowledgments
We thank the researchers Dr. Adrián Campelo for his counsel in osteocalcin expression evaluation and Dr. Pablo De Genaro for technical assistance for flow cytometry assays.
Funding
This work was supported by Universidad Nacional del Sur (UNS), Bahía Blanca, Argentina (grant number 24/B247); and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina, grant number PIP D-4061).
Author information
Authors and Affiliations
Contributions
S.B.C and M.J.S: conceptualization; methodology; investigation; writing original draft. M.C.C. and M.B.R: methodology; resources. V.L.M: writing-review and editing; supervision; project administration; funding acquisition.
Corresponding author
Ethics declarations
All the procedures involving animals were carried out in accordance with the guidelines published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Animal Care Use Committee approved the protocols employed in this study (Protocol number 028/2017).
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
UNS and CONICET had no role in the design, analysis, or writing of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Keypoints
The study provides cellular and molecular evidence of the osteoblastogenesis action of genistein.
The isoflavone enhances osteoblast differentiation marker expression.
Nitric oxide pathway plays a key role on bone phytoestrogen action.
Rights and permissions
About this article
Cite this article
Cepeda, S.B., Sandoval, M.J., Crescitelli, M.C. et al. The isoflavone genistein enhances osteoblastogenesis: signaling pathways involved. J Physiol Biochem 76, 99–110 (2020). https://doi.org/10.1007/s13105-019-00722-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-019-00722-3