Abstract
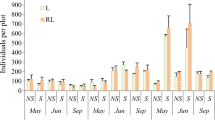
Management of the invasive Phragmites australis haplotype has focused on controlling its abundance in wetlands where it reduces biodiversity. However, little information is available on establishment of native communities and reinvasion by seed following removal using herbicides. The potential for reinvasion and development of native vegetation were evaluated using a seedbank assay and a vegetation survey along gradients from the channel edge to the marsh interior (0, 5 and 20 m distance) in three tidal freshwater marsh sites - Natural, treatment 6 months prior (Treated), and untreated (Phragmites). Recolonization potential from the seedbank was high with >18,500 seedlings m−2 in Treated samples. Richness and density of native species were low in the interior of Treated and Phragmites sites as compared to the Natural marsh. Few species were present in Treated site vegetation 11 months following treatment where P. australis litter comprised a large proportion of the cover. Results indicate that planting native vegetation to outcompete P. australis seedlings and total removal of P. australis to cut off the seed supply may be necessary for successful longer-term restoration and establishment of native species.






Similar content being viewed by others
Data Availability
Seedling data is in the Supplemental Table and vegetation data is available upon request.
References
Ailstock MS, Norman CM, Bushmann PJ (2001) Common reed Phragmites australis: control and effects upon biodiversity in freshwater nontidal wetlands. Restoration Ecology 9:49–59. https://doi.org/10.1046/j.1526-100x.2001.009001049.x
Amsberry L, Baker MA, Ewanchuk PJ, Bertness MD (2000) Clonal integration and the expansion of Phragmites australis. Ecological Applications 10:1110–1118
Armstrong J, Armstrong W, Beckett PM (1992) Phragmites australis: Venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytologist 120:197–207. https://doi.org/10.1111/j.1469-8137.1992.tb05655
Baldwin AH, Kettering KM, Whigham DF (2010) Seed banks of Phragmites australis-dominated brackish wetlands: relationships to seed viability, inundation, and land cover. Aquatic Botany 93:163–169
Bart D, Hartman JM (2002) Environmental constraints on early establishment of Phragmites australis in salt marshes. Wetlands 22:201–213
Bart D, Hartman JM (2003) The role of large rhizome dispersal and low salinity windows in the establishment of common reed, Phragmites australis, in salt marshes: new links to human activities. Estuaries and Coasts 26:436–443
Belzile F, Labbé J, LeBlanc M, Lavoie C (2010) Seeds contribute strongly to the spread of the invasive genotype of the common reed (Phragmites australis). Biological Invasions 12:2243–2250. https://doi.org/10.1007/s10530-009-9634-x
Benoit LK, Askins RA (1999) Impact of the spread of Phragmites on the distribution of birds in Connecticut tidal marshes. Wetlands 19:194–208. https://doi.org/10.1007/BF03161749
Brower JE, Zar JH, vonEnde CN (1998) Field and laboratory methods for general ecology, 4th edn. McGraw-Hill, Boston, Massachusetts, USA
Carlson ML, Kowalski KP, Wilcox DA (2009) Promoting species establishment in a Phragmites-dominated Great Lakes coastal wetland. Natural Areas Journal 29:263–280
Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth, England
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth, England
Colello J Jr (2013) Let’s go to the White City: a history of White City Amusement Park, Hamilton, NJ. Archway Publishing
Elsey-Quirk T, Leck MA (2015) Patterns of seed bank and vegetation diversity along a tidal freshwater river. Americam Journal of Botany 102:1996–2012
Galatowitsch SM, Anderson NO, Ascher PD (1999) Invasiveness in wetland plants in temperate North America. Wetlands 19:733–755. https://doi.org/10.1007/BF03161781
Galatowitsch SM, Larson DL, Larson JL (2016) Factors affecting post-control reinvasion by seed of an invasive species, Phragmites australis, in the Central Platte River, Nebraska. Biol Invasions 18:2505–2516. https://doi.org/10.1007/s10530-015-1048-3
Hallinger KD, Shisler JK (2009) Seed bank colonization in tidal wetlands following Phragmites control (New Jersey). Ecological Restoration 27:16–18
Hazelton ELG, Downard R, Kettenring KM, McCormick MK, Whigham DF (2018) Spatial and temporal variation in brackish wetland seedbanks: implications for wetland restoration following Phragmites control. Estuaries and Coasts 41:68–84
Hazelton ELG, Mozdzer TJ, Burdick DM, Kettenring KM, Whigham DF (2014) Phragmites australis management in the United States: 40 years of methods and outcomes. AoB PLANTS 6: plu001. https://doi.org/10.1093/aobpla/plu001
Hobbs RJ, Humphries SE (1995) An integrated approach to the ecology and management of plant invasions. Conservation Biology 9:761–770
Keller BEM (2000) Plant diversity in Lythrum, Phragmites, and Typha marshes, Massachusetts, U.S.a. Wetlands Ecology Management 8:391–401. https://doi.org/10.1023/a:1026505817409
Kettenring KM, Adams CR (2011) Lessons learned from invasive plant control experiments: a systematic review and meta-analysis. Journal of Applied Ecology 48:970–979
Kettenring KM, McCormick MK, Baron HM, Whigham DF (2011) Mechanisms of Phragmites australis invasion: feedbacks among genetic diversity, nutrients, and sexual reproduction. Journal of Applied Ecology 48:1305–1313. https://doi.org/10.1111/j.1365-2664.2011.02024.x
Kettenring KM, Mock KE (2012) Genetic diversity, reproductive mode, and dispersal differ between the cryptic invader, Phragmites australis, and its native conspecific. Biol Invasions 14:2489–2504. https://doi.org/10.1007/s10530-012-0246-5
Kettenring KM, Whigham D (2009) Seed viability and seed dormancy of non-native Phragmites australis in suburbanized and forested watershed of the Chesapeake Bay, USA. Aquatic Botany 91:199–204
Kettenring KM, Whigham D, Hazelton ELG, Gallagher SK, Weiner HM (2015) Biotic resistance, disturbance, and mode of colonization impact the invasion of a widespread, introduced wetland grass. Ecological Applications 25:466–480
Kettenring KM, Whigham DF (2018) The role of propagule type, resource availability, and seed source in Phragmites invasion in Chesapeake Bay wetlands. Wetlands 38:1259–1268. https://doi.org/10.1007/s13157-018-1034-5
Leck MA (1996) Germination of macrophytes from a Delaware River wetland. Biological Torrey Botanical Club 123:48–67
Leck MA (2003) Seed-bank and vegetation development in a created tidal freshwater wetland on the Delaware River, Trenton, New Jersey, USA. Wetlands 23:310–343
Leck MA (2013) Dispersal potential of a tidal river and colonization of a created tidal freshwater marsh. AoB PLANTS (Oxford Univ. Press online journal)(special issue regarding Phragmites australis). (pls050) AoB PLANTS 5: pls050. https://doi.org/10.1093/aobpla/pls050
Leck MA, Graveline KJ (1979) The seed bank of a freshwater tidal marsh. American Journal of Botany 66:1006–1015
Leck MA, Simpson RL (1987) Seed bank of a freshwater tidal wetland: turnover and relationship to vegetation change. Am J Botany 74(3):360–370
Leck MA, Simpson RL (1994) Tidal freshwater wetland zonation: seed and seedling dynamics. Aquatic Botany 47:61–75
Leck MA, Simpson RL (1995) Ten-year seed bank and vegetation dynamics of a tidal freshwater wetland. American Journal of Botany 82:1547–1557
Leck MA, Simpson RL, Whigham DF, Leck CF (1988) Plants of the Hamilton marshes, a Delaware River freshwater tidal wetland. Bartonia 54:1–18
Marks M, Lapin B, Randall J (1994) Phragmites australis (P. communis): threats, management and monitoring. Natural Areas Journal 14:285–294
Martin LJ, Blossey B (2013) The runaway weed: costs and failures of Phragmites australis management in the USA. Estuaries and Coasts 36:626–632
Mauchamp A, Blanch S, Grillas P (2001) Effects of submergence on the growth of Phragmites australis seedlings. Aquatic Botany 69:147–164
McCormick MK, Kettenring KM, Baron HM, Whigham DF (2010) Extent and reproductive mechanisms of Phragmites australis spread in brackish wetlands in Chesapeake Bay, Maryland (USA). Wetlands 30:67–74
Meyerson LA, Saltonstall K, Windham L, Kiviat E, Findlay S (2000) A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecology and Management 8:89–103
Minchinton TE, Bertness MD (2003) Disturbance-mediated competition and the spread of Phragmites australis in a coastal marsh. Ecological Applications 13:1400–1416
Minchinton TE, Simpson JC, Bertness MD (2006) Mechanisms of exclusion of native coastal marsh plants by an invasive grass. Journal of Ecology 94:342–354
Myers JH, Simberloff D, Kuris AM, Carey JR (2000) Eradication revisited: dealing with exotic species. Trends Ecology Evolution 15:316–320
Neff KP, Rusello K, Baldwin AH (2009) Rapid seed bank development in restored tidal freshwater wetlands. Restoration Ecology 17(4):539–548
Padgett DJ (2007) A monograph of Nuphar (Nymphaeaceae). Rhodora 109:1–95
Parker VT, Leck MA (1985) Relationships of seed banks to plant distribution patterns in a freshwater tidal wetland. American Journal of Botany 72:161–174
Reid AM, Morin L, Downey PO, French K, Virtue JG (2009) Does invasive plant management aid the restoration of natural ecosystems? Biological Conservation 142:2342–2349
Rohal CB, Cranney C, Kettenring KM (2019) Abiotic and landscape factors constrain restoration outcomes across spatial scales of a widespread invasive plant. Frontiers in Plant Science 10:481. https://doi.org/10.3389/fpls.2019.00481
Rooth JE, Stevenson JC, Cornwell JC (2003) Increased sediment accretion rates following invasion by Phragmites australis: the role of litter. Estuaries Coasts 26:475–483
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proceedings of the Natural Academy of Sciences of the United States of America 99:2445–2449. https://doi.org/10.1073/pnas.032477999
Saltonstall K, Lambert A, Meyerson L (2010) Genetics and reproduction of common (Phragmites australis) and giant reed (Arundo donax). Invasive Plant Science and Management 3:495–505. https://doi.org/10.1614/IPSM-09-053.1
Thompson K, Bakker JP, Bekker RM (1997) The soil seed banks of North West Europe: methodology, density, and longevity. Cambridge University Press, Cambridge
USDA ITIS (2020) https://www.208itis.gov/
van der Valk AG, Pederson RL (1989) Seedbanks and the management and restoration of natural vegetation. In: Leck MA (ed) 2012 Ecology of soil seedbanks. Elsevier
Whigham DF, Simpson RL (1975) Ecological studies of the Hamilton marshes, progress report for the period June 1974–January 1975. Rider College, Trenton, New Jersey, USA
White TJ (2014) The effects of Phragmites australis litter on seed emergence in the Erie-Huron corridor, Michigan. Thesis. Wayne State University, East Lansing, Michigan, USA
Windham L, Lathrop RG (1999) Effects of Phragmites australis (common reed) invasion on aboveground biomass and soil properties in brackish tidal marsh of the Mullica river, New Jersey. Estuaries 22:927–935. https://doi.org/10.2307/1353072
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Critical Reviews Plant Sciences. 23:431–452
Acknowledgements
Logistical support was provided by Mercer County Park Commission (Emily Blackman, Alexandria Kosowski, Alex Rivera, Jenn Rogers, Kelly Rypkema, and Jillian Stark) and NJ Department of Transportation (Jason Nowak); field help was provided by Kirk Raper and Nick Alpeza; Jen Scheibner and Alexis Windecker ably assisted with greenhouse duties. Rider University provided greenhouse space. Mark Gallagher from Princeton Hydro, Princeton, NJ provided treatment information. This research was funded by NSF Coastal SEES 1325466. We appreciate the careful reviews of two anonymous reviewers.
Funding
This research was funded by NSF Coastal SEES 1325466.
Author information
Authors and Affiliations
Contributions
T. Elsey-Quirk and M.A. Leck conceived of, designed and implemented the study and wrote the manuscript. M.A. Leck maintained the greenhouse and identified the seedlings. Field surveys were conducted by T. Elsey-Quirk and M.A Leck. T. Elsey-Quirk analyzed the data.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
None.
Ethics Approval
Not applicable.
Consent to Participate
T. Elsey-Quirk and M.A. Leck consent to participate.
Consent for Publication
These results have not been published elsewhere.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elsey-Quirk, T., Leck, M.A. High Reinvasion Potential of Phragmites australis in a Delaware River (USA) Tidal Freshwater Marsh Following Chemical Treatment: the Role of the Seedbank. Wetlands 41, 12 (2021). https://doi.org/10.1007/s13157-021-01398-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-021-01398-6